Antigen Specific T Cells for Assessing Immune Modulating Therapeutics
Antigen-specific T cells are essential for evaluating immune-modulating therapies, but sourcing them for research is challenging. This blog highlights the development of ‘Thaw & Go’ transgenic TCR-T cell banks and methods to expand endogenous antigen-specific T cells, providing ready-to-use resources for high-throughput assessment of immune modulators and advancing drug discovery efforts.

|
October 22, 2025
|
4 min read
Antigen-specific T cells play a key role in the adaptive immune system, as they can identify and eliminate cells presenting their specific antigen. Their targeted nature makes them valuable for testing immune-modulating therapies designed to either boost or suppress immune activity in diseases like cancer and autoimmunity. A major obstacle in evaluating antigen-specific T cell responses is having readily available cells to study the impact of immune modulators in real time. To address this, we have developed several well-characterised transgenic ‘Thaw & Go’ TCR-T cell banks that facilitate the evaluation of new immune modulators.
Generation of Transgenic TCR-T Cells
Donors were selected based on HLA genotype compatibility with the antigen of interest, ensuring TCR-T cells will recognise their specific antigen when presented by the specific HLA genotype.
CD4+ or CD8+ T cells were isolated from cryopreserved PBMC (supplied by BioIVT, LLC) and transduced with a lentiviral vector encoding the TCR construct. An upstream tag enabled selection of successfully transduced cells. The cells were expanded using a proprietary protocol, and then characterised and cryopreserved.
Characterisation of the cells included transgenic TCR expression, and T cell exhaustion and activation markers.

Functional Assessment of TCR-T Cells
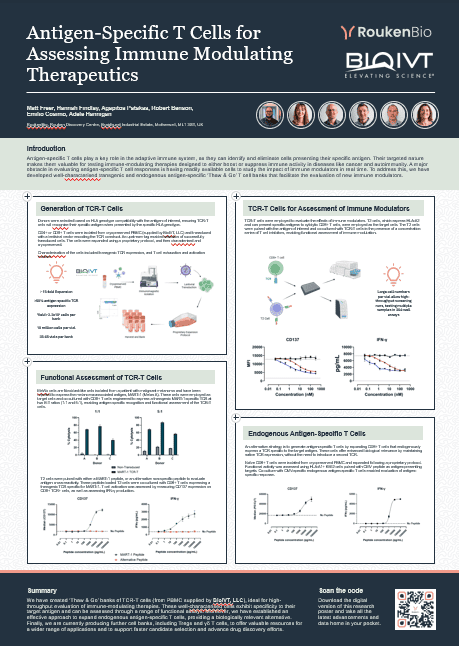
MeWo cells are fibroblast-like cells isolated from a patient with malignant melanoma and have been reported to express the melanoma-associated antigen, MART-1 (Melan A). These cells were employed as target cells and co-cultured with CD8+ T cells engineered to express a transgenic MART-1-specific TCR at two E:T ratios (1:1 and 5:1), enabling antigen specific recognition and functional assessment of the TCR-T cells.
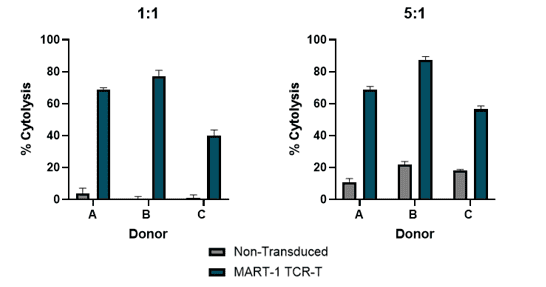
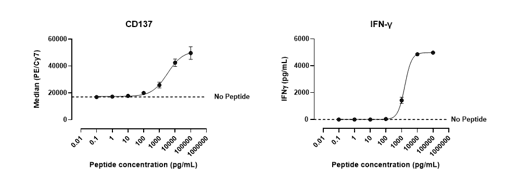
T2 cells were pulsed with either a MART-1 peptide, or an alternative non-specific peptide to evaluate antigen cross-reactivity. These peptide-loaded T2 cells were co-cultured with CD8+ T cells expressing a transgenic TCR specific for MART-1. T cell activation was assessed by measuring CD137 expression on CD8+ TCR+ cells, as well as assessing IFN-γ production.
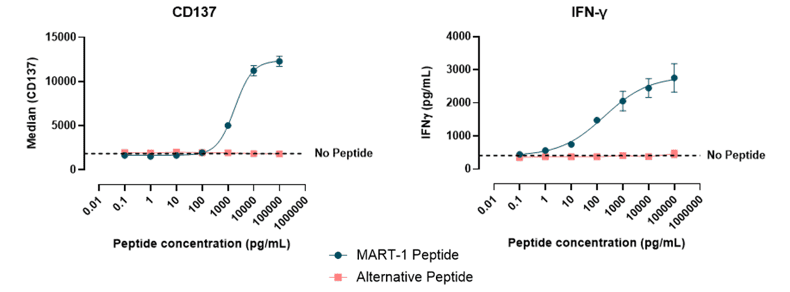
TCR-T Cells for Assessment of Immune Modulators
TCR-T cells were employed to evaluate the effects of immune modulators. T2 cells, which express HLA-A2 and can present specific antigens to cytolytic CD8+ T cells, were employed as the target cells. The T2 cells were pulsed with the antigen of interest and co-cultured with TCR-T cells in the presence of a concentration series of T cell inhibitors, enabling functional assessment of immune modulation.
Endogenous Antigen-Specific T-Cells
An alternative strategy is to generate antigen-specific T cells by expanding CD8+ cells that endogenously express a TCR specific to the target antigen. These cells offer enhanced biological relevance by maintaining native TCR expression, without the need to introduce a second TCR.
Naïve CD8+ T cells were isolated from cryopreserved PBMC and expanded following a proprietary protocol. Functional activity was assessed using HLA-A1+ K562 cells pulsed with CMV peptide as antigen-presenting targets. Co-culture with CMV-specific endogenous antigen-specific T cells enabled evaluation of antigen-specific response.
Summary
We have addressed the challenge of obtaining ready-to-use antigen-specific T cells by creating ‘Thaw & Go’ banks of transgenic TCR-T cells (from PBMC supplied by BioIVT, LLC), ideal for high-throughput evaluation of immune-modulating therapies. These well-characterised cells exhibit specificity to their target antigen and can be assessed through a range of functional assays. Moreover, we have established an effective approach to expand endogenous antigen-specific T cells, providing a biologically relevant alternative. Finally, we are currently producing further cell banks, including Tregs and γδ T cells, to offer valuable resources for a wider range of applications and to support faster candidate selection and advance drug discovery efforts.
Special thanks to BioIVT who helped in the creation of our collaborative research poster.
Figures were created using BioRender.
Join our community of curious minds on LinkedIn
🗓️ Stay informed with our monthly scientific newsletter, published on LinkedIn on the last Wednesday of each month.
These editions bring you the latest in drug development breakthroughs, industry trends, and expert insights from the brilliant minds at RoukenBio.
Subscribe today on LinkedInReady to accelerate your research?
See how our specialised expertise can fast-track your progress. Dive into two compelling CAR-T case studies and find out how our advanced platforms and comprehensive capabilities empower your cutting-edge cell therapy projects.
Access the CAR-T slides