Autoimmunity
Autoimmune diseases arise when a dysregulated immune system mistakenly targets and attacks the body, leading to chronic inflammation and tissue damage. The complex mechanisms underlying autoimmunity have revealed novel targets for modern therapeutic interventions that modulate the immune response, moving beyond the generalised immunosuppression of traditional therapies. At RoukenBio, we have the knowledge and capabilities to screen and assess your therapeutics against these novel targets using physiologically relevant autoimmunity assays.

Our autoimmunity bioassays
At RoukenBio, we specialise in offering a wide range of off-the-shelf and bespoke assays for studying the immune processes responsible for driving autoimmunity. These assays cover a range of cell types including those that exacerbate inflammation, such as T cells, to those that can actively suppress it, such as regulatory T cells (Tregs) and macrophages. Importantly, our expertise and flexible approach allow us to study the impact of your therapeutics in a number of areas including activation, suppression, cell phenotype polarisation and immune cell targeted depletion.
Example assays include:
- Mixed Lymphocyte Reactions assay
- T cell Activation assay
- Treg Suppression assay
- T cell Polarisation assay
- B cell Depletion assay
- T cell Depletion assay
Explore the diverse range of cell types and assays that RoukenBio offers focusing on autoimmune research.
Cells in Autoimmunity Assays
T cells
Tregs
Macrophages
T cells
T cells play a crucial role in the development and progression of autoimmune diseases. In a healthy immune system, T cells help protect the body by directly killing pathogen infected cells and by regulating the immune response. However, in autoimmunity, this system malfunctions, causing T cells to mistakenly target the body's own tissues.
This misdirected attack is often due to a breakdown in the mechanisms that normally ensure self-tolerance, such as thymic selection and peripheral tolerance checkpoints. When these mechanisms fail, self-reactive T cells can proliferate and initiate an immune response against the body's own cells, leading to chronic inflammation and tissue damage.
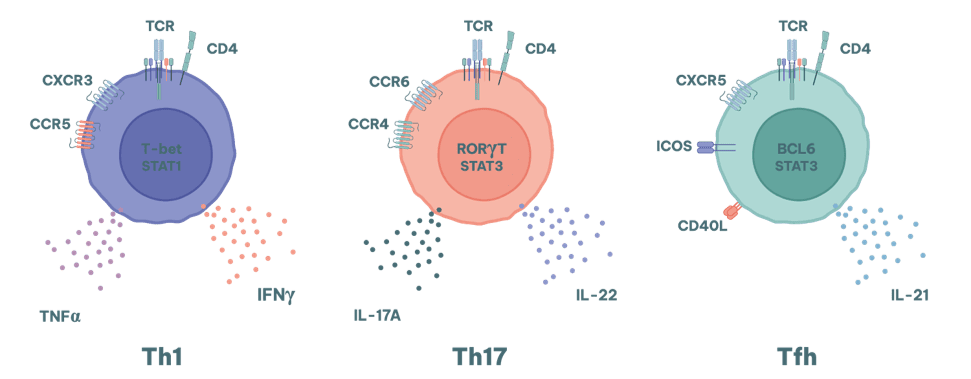
Different subsets of T cells contribute in different ways to various autoimmune conditions.
- Th1 cells are involved in cell-mediated immunity in organ specific diseases such as type 1 diabetes and multiple sclerosis.
- Th17 cells are strongly associated with chronic inflammation and tissue damage in diseases such as rheumatoid arthritis (RA), psoriasis and inflammatory bowel disease (IBD).
They are notably relevant to diseases where neutrophil recruitment is a key pathologic feature. Where the production of high affinity autoantibodies exacerbates disease severity (e.g. SLE and RA), the activity of Tfh cells is also a key consideration.
RoukenBio offers an array of different autoimmunity assay types that can be used to screen drugs and their effects upon T cell differentiation, polarisation and cytotoxicity.
T cells

T cells play a crucial role in the development and progression of autoimmune diseases. In a healthy immune system, T cells help protect the body by directly killing pathogen infected cells and by regulating the immune response. However, in autoimmunity, this system malfunctions, causing T cells to mistakenly target the body's own tissues.
This misdirected attack is often due to a breakdown in the mechanisms that normally ensure self-tolerance, such as thymic selection and peripheral tolerance checkpoints. When these mechanisms fail, self-reactive T cells can proliferate and initiate an immune response against the body's own cells, leading to chronic inflammation and tissue damage.
Different subsets of T cells contribute in different ways to various autoimmune conditions.
- Th1 cells are involved in cell-mediated immunity in organ specific diseases such as type 1 diabetes and multiple sclerosis.
- Th17 cells are strongly associated with chronic inflammation and tissue damage in diseases such as rheumatoid arthritis (RA), psoriasis and inflammatory bowel disease (IBD).
They are notably relevant to diseases where neutrophil recruitment is a key pathologic feature. Where the production of high affinity autoantibodies exacerbates disease severity (e.g. SLE and RA), the activity of Tfh cells is also a key consideration.
RoukenBio offers an array of different autoimmunity assay types that can be used to screen drugs and their effects upon T cell differentiation, polarisation and cytotoxicity.
Tregs
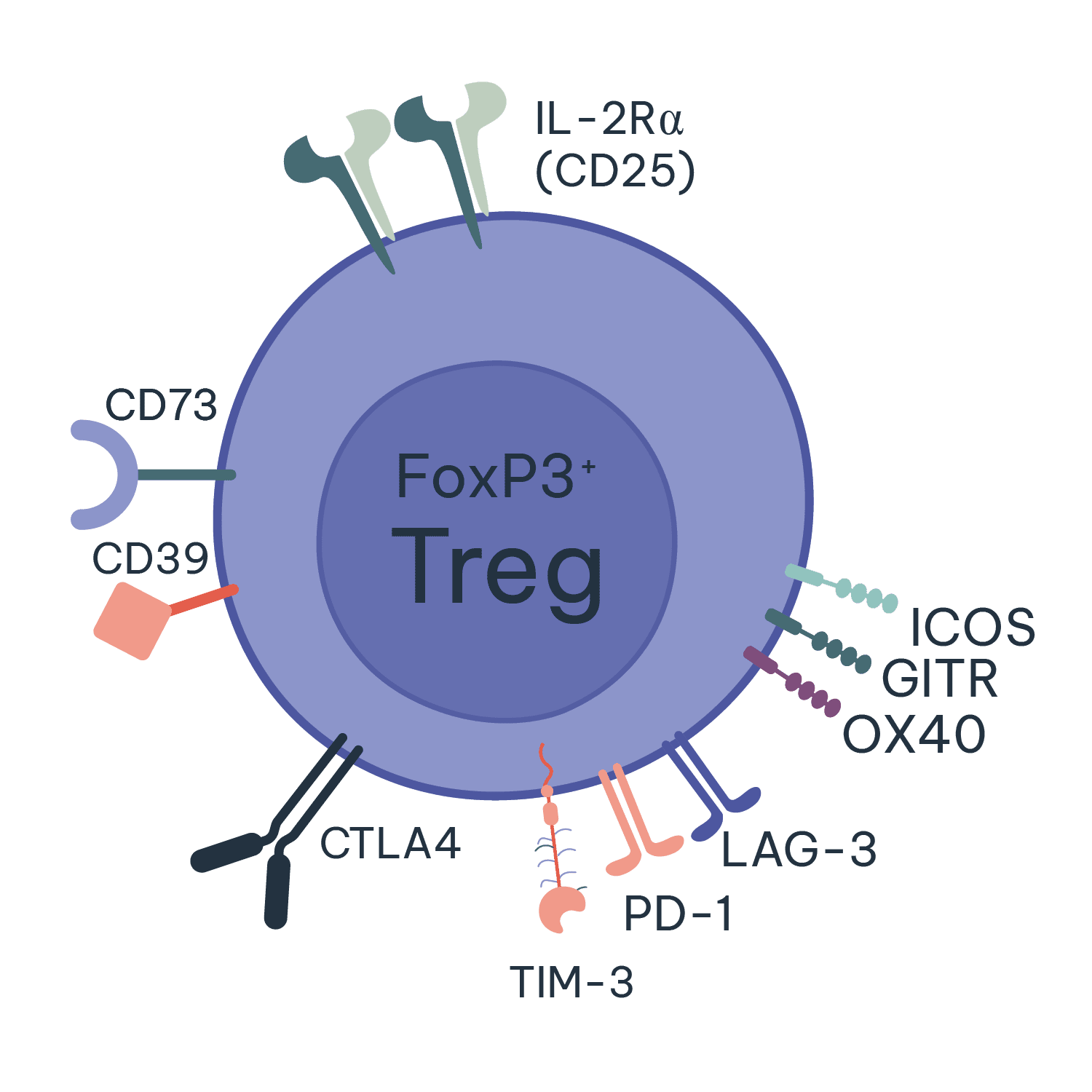
Regulatory T cells (Tregs) are essential for maintaining immune system balance and preventing autoimmunity. They achieve this by suppressing the activity of other immune cells, helping to control and resolve inflammatory immune responses whilst also preventing reactions to self-antigens.
In autoimmune diseases, the malfunction or deficiency of Tregs can lead to a loss of self-tolerance, allowing self-reactive T cells to proliferate and cause tissue damage. This dysregulation can result in chronic inflammation and the progression of autoimmune disorders. Enhancing the function or number of Tregs is a promising therapeutic strategy for treating autoimmune diseases, as it can help restore immune balance and reduce pathological immune responses. At RoukenBio, our experts use our Treg induction and suppression assays to help screen and test your Treg targeting therapeutics.
Macrophages
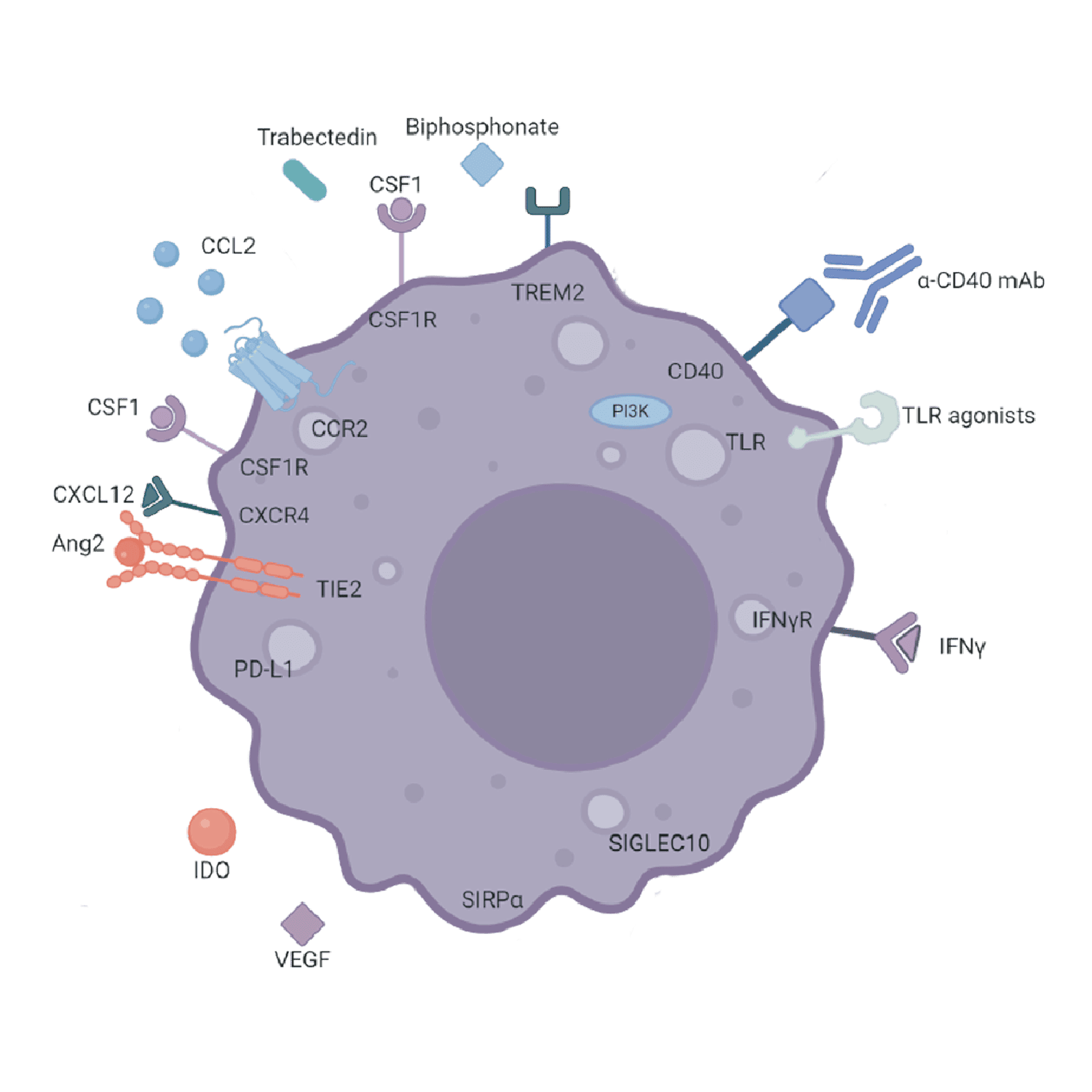
Macrophages are key players in the immune system, having both pro- and anti-inflammatory roles depending upon the specific subtype. In autoimmunity, macrophages can contribute to disease pathogenesis through their roles in inflammation and tissue damage. They produce various cytokines and chemokines that can either promote or suppress immune responses, depending on the signals they receive from their environment.
In autoimmune diseases, macrophages in the affected tissues exacerbate inflammation and tissue destruction through their role as antigen presenting cells, which induce the activation of autoreactive T cells. In addition, they release pro-inflammatory cytokines like TNF-α and IL-1, which further amplify the immune response and contribute to chronic inflammation.
However, macrophages also have the potential to suppress autoimmune responses and promote tissue repair. Certain subsets of macrophages, known as M2 macrophages, can produce anti-inflammatory cytokines and growth factors that help resolve inflammation and support tissue repair or, in some instances, drive fibrosis. At RoukenBio, we have a number of macrophage-based assays that can examine macrophage differentiation, activation and suppression.
Autoimmunity assays and tools
Mixed Lymphocyte Reaction Assay
Treg Suppression Assays
Macrophage Polarisation Assays
B Cell Depletion Assays
T Cell Depletion Assays
Mixed Lymphocyte Reaction Assay
Mixed lymphocyte reactions (MLR) are assays in which T cells and antigen presenting cells (APCs) from two genetically mismatched (allogenic) donors are incubated together to induce T cell activation. These assays are commonly used to assess the ability of biologics or small molecules to directly or indirectly alter T cell activation, proliferation and cytokine secretion.
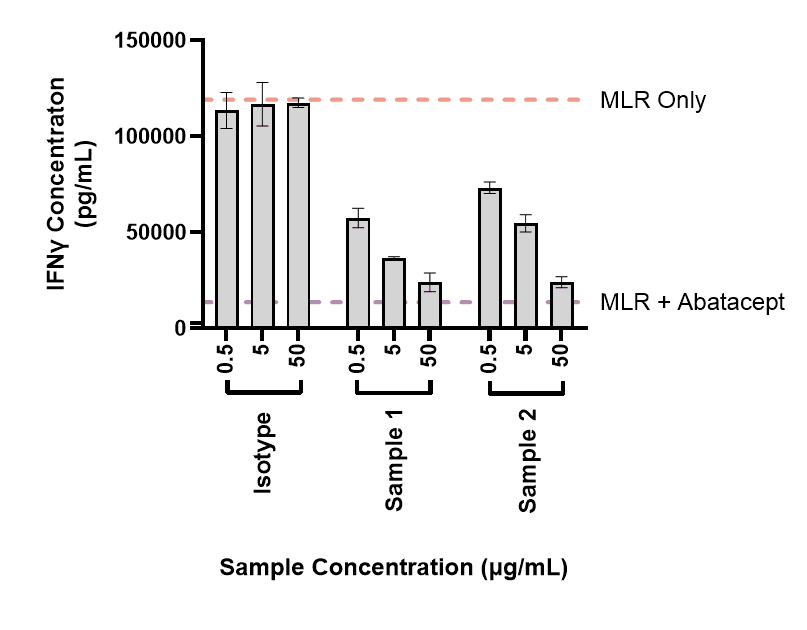
In the context of autoimmunity, an MLR is a useful assay for determining the ability of molecules to suppress T cell responses. The assay can be modified in various ways to expand the assay window allowing the impact of therapeutics to be more closely examined. This includes molecules like the IgG1 fusion protein abatacept, which is used for the treatment of RA. An example of data from this type of assay is shown:
Mixed Lymphocyte Reaction Assay
Mixed lymphocyte reactions (MLR) are assays in which T cells and antigen presenting cells (APCs) from two genetically mismatched (allogenic) donors are incubated together to induce T cell activation. These assays are commonly used to assess the ability of biologics or small molecules to directly or indirectly alter T cell activation, proliferation and cytokine secretion.
In the context of autoimmunity, an MLR is a useful assay for determining the ability of molecules to suppress T cell responses. The assay can be modified in various ways to expand the assay window allowing the impact of therapeutics to be more closely examined. This includes molecules like the IgG1 fusion protein abatacept, which is used for the treatment of RA. An example of data from this type of assay is shown:

Treg Suppression Assays
Treg suppression assays are used to evaluate the ability of Tregs to suppress the proliferation and activity of T cell subtypes, such as CD4+ or CD8+ T cells. In these assays, T cells are labelled with a cell tracker dye and incubated with Tregs at different ratios in the presence of a stimulant. The extent to which Tregs inhibit the proliferation and cytokine production of the T cells is then assessed via flow cytometry and ELISA. These assays are well suited to testing the ability of therapeutics to induce Tregs or to enhance their responses. An example of a Treg suppression assay in which different ratios of Tregs are incubated with stimulated conventional T cells (Tresp) is shown below:
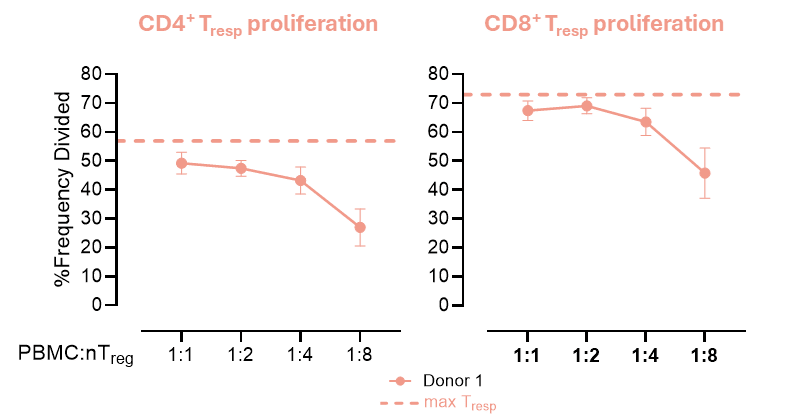
Macrophage Polarisation Assays
In autoimmunity research, macrophage polarisation assays are used to study the functional states of macrophages, specifically their polarisation from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype. These assays involve culturing macrophages with a therapeutic molecule under specific conditions to determine if M1 macrophages can be inhibited or redirected to an anti-inflammatory state. Phenotypic changes are assessed via altered cytokine production and changes in the expression of different surface markers. An example of flow cytometry data that would be used to determine macrophage polarisation states is shown below:
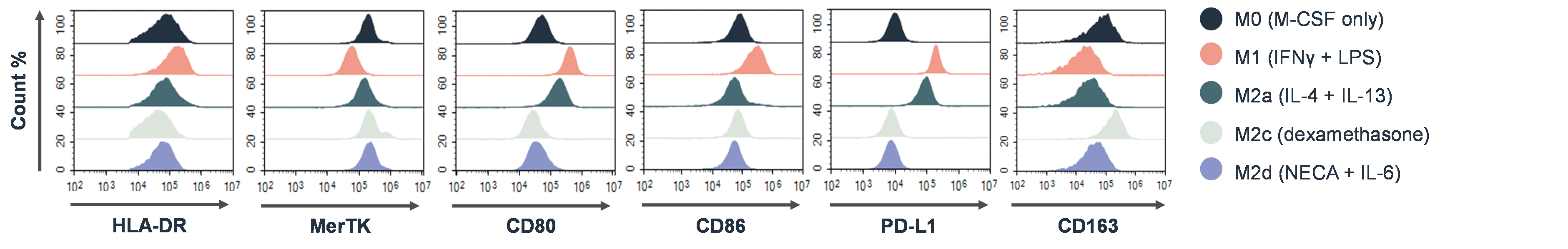
B Cell Depletion Assays
B cell depletion assays are used to evaluate the effectiveness of therapies that target B cells in cancers as well as autoimmune diseases. Current therapies used for this purpose include rituximab, which removes the autoantibody producing B cells in RA. In B cell depletion assays PBMCs or bone marrow are incubated with therapeutic agents for a pre-determined time. The reduction in specific B cell populations is then measured via flow cytometry. An example of the type of data generated from a B cell depletion assay using the CD3xCD19 T cell engager blinatumomab is shown here:
T Cell Depletion Assays
Specific rare autoreactive T cell populations represent attractive targets in T cell–driven autoimmune diseases. RoukenBio can assess T cell depletion using either pan T cells or defined rare T cell populations within Fc-mediated effector function assays (ADCC, ADCP) and TDCC.
In the ADCC-driven example presented, target T cells are incubated with purified autologous NK cells for 24 hours in the presence of a Fc-competent therapeutic antibody, with pathogenic T cell depletion quantified by flow cytometry. The data demonstrates dose-dependent depletion of the target T cell population, while all other T cell populations remained intact.
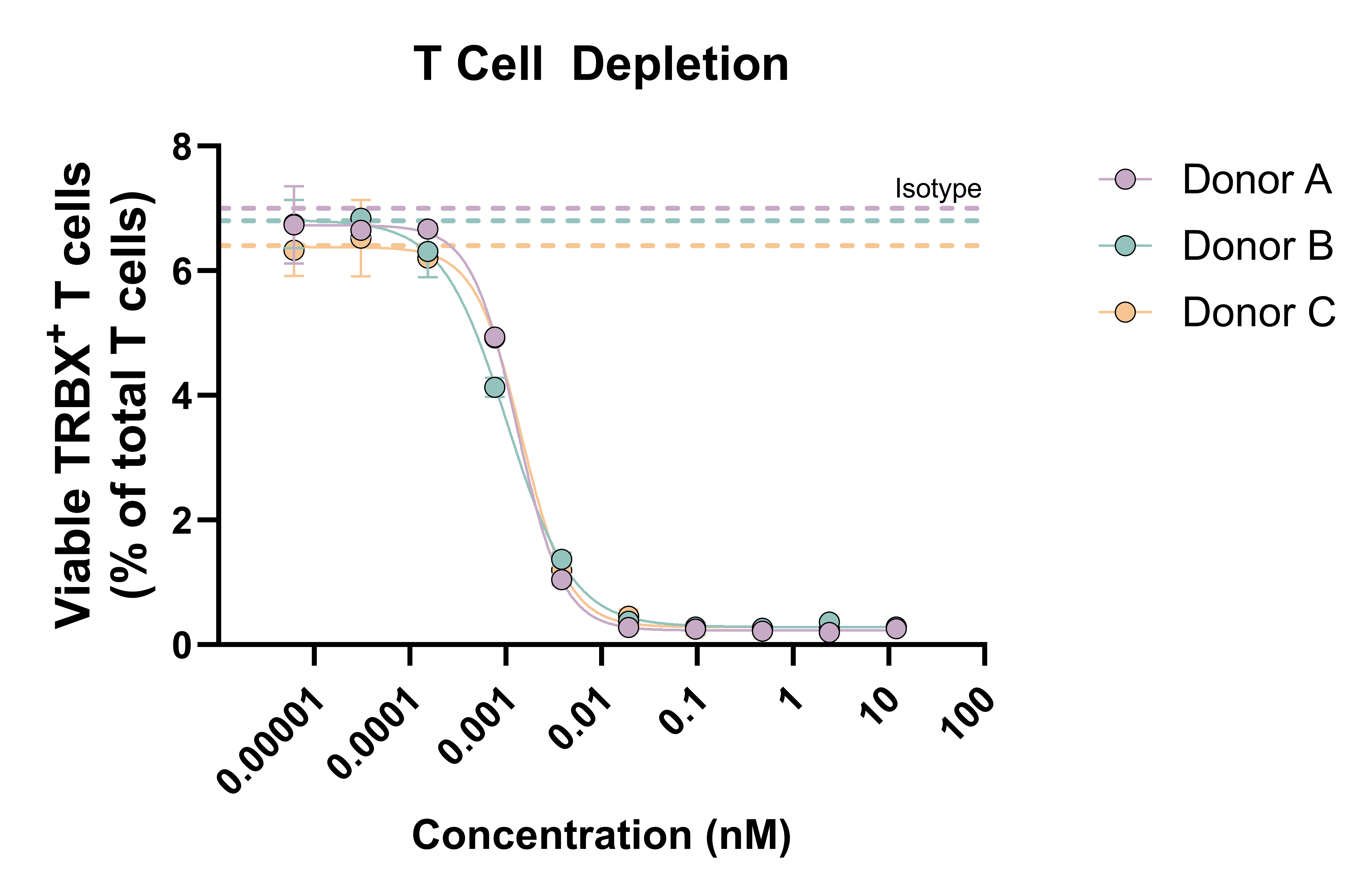
Pan T cells were incubated with purified NK cells (autologous donor) for 24 hours and T cell depletion was assessed by flow cytometry (% target expressing)
Why choose RoukenBio for autoimmunity assays?
At RoukenBio, the Immunology CRO redefined, our profound knowledge in translational immunology and autoimmune pathophysiology enables us to support the development of innovative therapies that modulate immune responses to restore balance and alleviate disease symptoms. Our advisory collaborative approach, backed by decades of collective experience of our brilliant minds, has led to the creation of an array of in vitro assay systems that recreate autoimmune environments, closely mirroring physiological processes.
Extensive autoimmune expertise
We leverage our brilliant minds’ academic and industrial knowledge of autoimmunity to answer your specific questions.
Bespoke assay design
Our assays are tailored to your specific needs.
State-of-the-art technologies
Advanced flow cytometry, impedance-based systems, bioassays, and reporter-based technologies.
Propel drug discovery with RoukenBio's autoimmunity assays
Let us help accelerate your autoimmunity research and therapeutic development with our bioassay expertise supporting your next breakthrough in autoimmunity treatment.
Access our Autoimmune assay slides
