Cell Therapy
We support the development of cutting-edge cell therapies, offering comprehensive services from vector design and cell engineering to in vitro efficacy and safety testing. Our advanced 3D Cell Platforms and Immune Cell Platforms enable precise and effective development of cell therapies.

With unparalleled expertise in immune cell therapies and cutting-edge analytics, we are your trusted partner for advancing immune cell therapy projects across all formats, including CAR-T, TCR-T, CAR-NK, CAR-γδ T, and CAR-Macrophages.
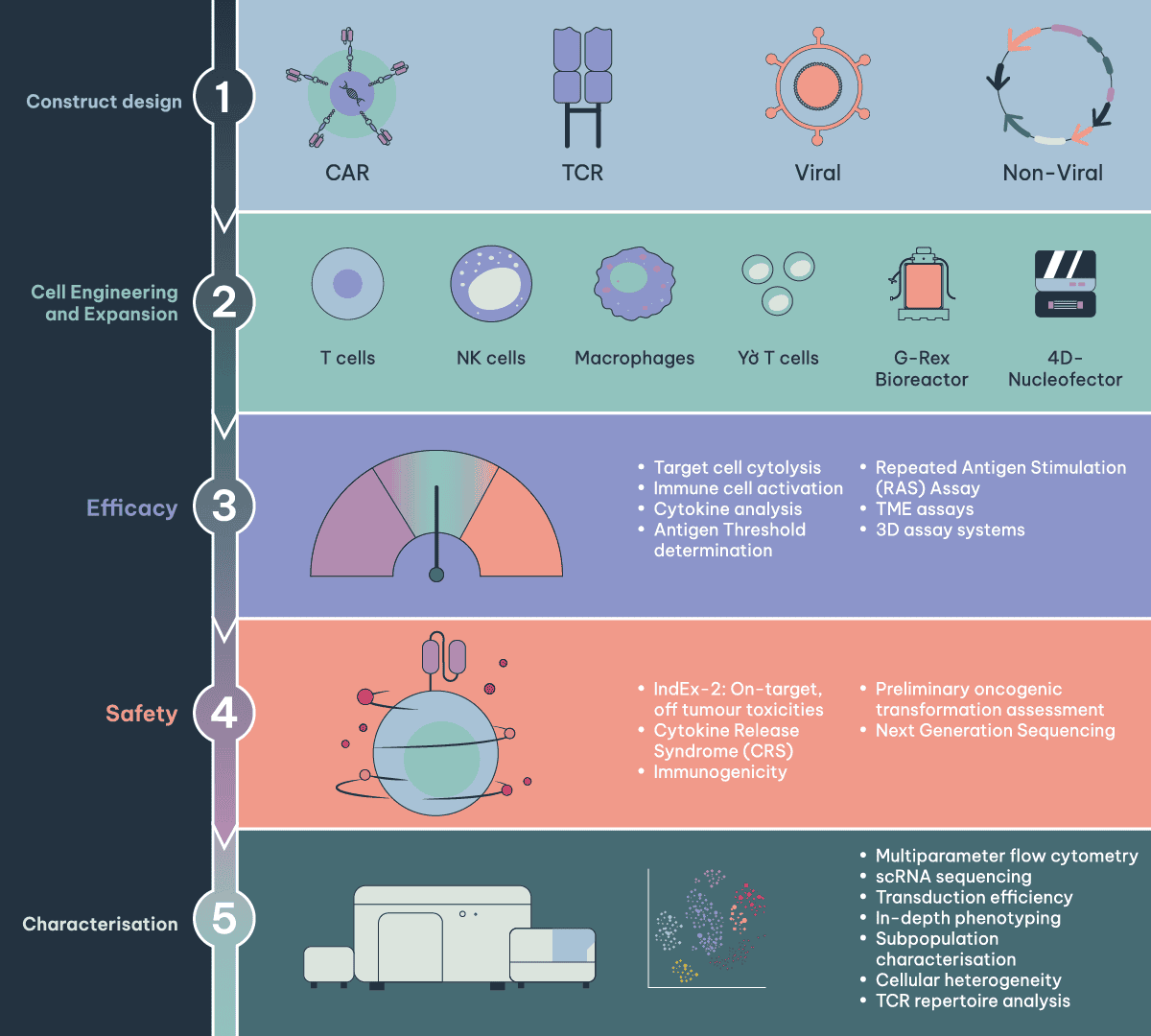
We deliver tailored solutions, leveraging the latest innovations and industry best practices in:
Construct design
Cell Engineering and Expansion Optimisation
Efficacy
Safety
Characterisation
Construct design
By utilising cutting-edge molecular biology, we can guide you through construct design, tailored to your specific needs. With extensive experience in both conventional CAR and TCR designs and advanced structures like conditional, bispecific, dual, and logic-gated CARs, we ensure your project’s success. Our expertise spans viral and non-viral vector delivery methods, and we use advanced technologies like CRISPR (MAD7) for precise genetic modifications. We understand immune cell biology, integrating this knowledge into construct design to maximise efficacy and safety.
Construct design
By utilising cutting-edge molecular biology, we can guide you through construct design, tailored to your specific needs. With extensive experience in both conventional CAR and TCR designs and advanced structures like conditional, bispecific, dual, and logic-gated CARs, we ensure your project’s success. Our expertise spans viral and non-viral vector delivery methods, and we use advanced technologies like CRISPR (MAD7) for precise genetic modifications. We understand immune cell biology, integrating this knowledge into construct design to maximise efficacy and safety.
Cell Engineering and Expansion Optimisation
Developing effective CAR cell therapies demands precise immune cell engineering methodologies to enhance therapeutic potential. We have extensive expertise in genetic modification, expansion, and differentiation of diverse immune cells, including αβ and γδ T cells, natural Tregs, TILs, NK cells, and macrophages. We specialise in both viral and non-viral methods, such as transposons and RNA, offering custom solutions tailored to your project. Our expansion strategies are designed to optimise activation, transduction, and growth conditions, ensuring the final product meets your desired functional characteristics.
Efficacy
We offer a range of assay systems and tools to assess on-target efficacy and Mechanism of Action (MOA) of immune cell therapies and can develop custom solutions to support unique development requirements. The extended portfolio of assay systems and tools include:
- High throughput construct screening employing well-established reporter systems such as the Jurkat NFAT-RE Luc.
- An extended portfolio of target cell lines, stemming from cancer cell lines to primary patient derived tissues. Furthermore, our ground-breaking IndEx-2 inducible cell line system allows assessment of the impact of target antigen density on the efficacy of immune cell therapeutics.
- Target cell cytolysis assays employing a variety of readouts including:
- Electrical impedance-based Real Time Cell Analysis (RTCA), that provides data-rich, label free kinetic measurements of cell viability and proliferation.
- Flow cytometry based high-content analysis of tumour death, providing mechanistic details of cell death.
- Luminescent based, high-throughput measurements of cell killing.
- In depth cytokine analysis using:
- Luminex® or cytometric bead array technologies for comprehensive multiplex analysis of cytokine expression.
- AlphaLISA for no wash, rapid, highly sensitive and accurate quantification.
- ELISA for cost effective analysis of a focused number of cytokines.
- Intracellular cytokine analysis to link cytokine production to cellular phenotype.
- Advanced in vitro anti-tumour efficacy assays that emulate the microenvironment that immune cell therapies need to exert their function:
- Repeated Antigen Stimulation (RAS) Assay: Evaluate the efficacy of CAR-T and TCR-T therapies under "antigen stress" by repeatedly exposing effector cells to fresh target cells, assessing retention, modification, or loss of function using advanced techniques like impedance-based RTCA, multiparameter flow cytometry, and multiplexed cytokine analysis.
- Tumour Microenvironment (TME) Assays: Ensure the effectiveness of cell therapies by testing cytotoxicity and functionality under harsh conditions, including exposure to soluble immunomodulators (TGFβ, IL-10, Adenosine, PGE2) and coculture with immunosuppressive cells (e.g. M2 macrophages and Treg cells), simulating the tumour microenvironment.
- Advanced 3D Assay Systems: Gain access to scalable, high-throughput 3D assays with spheroids, patient-derived tumoroids, or tissue explants (PDTEs) through RoukenBio’s partnership with Screenin3D, utilising cutting-edge microfluidic lab-on-a-chip technology.
Safety
Evaluating the safety and efficacy of CAR cell therapies involves extensive in vitro and in vivo testing. This includes assessing the potential for on-target, off-tumour toxicity, Cytokine Release Syndrome (CRS), immunogenic potential and cytokine-independent proliferation.
- Evaluating on-target, off-tumour toxicities in immune cell therapies is essential for patient safety and is a critical requirement in FDA preclinical development guidelines. Rouken Bio’s IndEx-2 system is the only in vitro system that allows precise determination of antigen threshold of initiation of effector function. Employing cell lines that allow inducible and titratable expression of one or two antigens of interest and high content assay systems we can determine thresholds of activation for different MOAs (e.g. cytolysis, cytokine production and proliferation). By correlating activity thresholds with physiological and pathological tissue expression we can provide a preliminary risk profile early in the development process.
- CRS is a critical safety concern in immune cell therapies. We expertly evaluate CRS during preclinical development by employing multiplexed cytokine analysis to assess cytokine expression profiles, correlating them with antigen density and construct design.
- Assessing immunogenicity is vital to ensure the safety and efficacy of immune cell therapies, as it directly impacts patient response and long-term treatment success. At Rouken Bio, we offer a comprehensive portfolio of assays to evaluate immunogenicity, whether on recombinant chimeric proteins and TCRs or the full cell therapy products. Our well-characterised cohort of HLA-typed donors enables precise immunogenicity studies, including alloreactivity assessment using mixed lymphocyte reaction (MLR) assays for allogeneic therapies.
- Most cell therapy workflows rely on random integration to introduce CAR or TCR into the host cell genome, which carries the risk of insertional mutagenesis and potential oncogenic transformation. To assess this risk, we evaluate the ability of genetically modified cells to proliferate without cytokine or antigen stimulus. If these cells show abnormal proliferation compared to unedited cells, we conduct further analysis using next-generation sequencing (NGS) to thoroughly investigate the oncogenic potential and ensure the safety of the cell therapy product.
Characterisation
Immune cell products are complex and heterogeneous and require extensive characterisation to ensure safety and potency characteristics. At RoukenBio we employ flow cytometry or single cell RNA sequencing (scRNA-seq) to provide unparalleled, in-depth characterisation of the cell therapy product.
- We employ multiparametric flow cytometry to assess transduction efficiency, composition and phenotypic characteristic of the final product. We have advanced flow cytometers that allow the assessment of up to 27 parameters and routinely develop 15 parameter panels. In the absence of anti-idiotypic antibodies we have extensive experience in employing recombinant antigens to directly assess expression of the CAR.
- To further enhance the characterization of immune cell therapy products, we utilize scRNA-seq with scalable combinatorial barcoding technology. This powerful approach allows us to capture the full spectrum of gene expression at the single-cell level, including TCR repertoire analysis, providing detailed insights into cellular heterogeneity, immune cell function, and potential off-target effects. By leveraging scRNA-seq, we can more accurately assess the safety, efficacy, and overall quality of immune cell therapies.
Connect with us today to explore how our expertise can drive the success of your immune cell therapy project. Let’s transform innovative ideas into impactful therapies together.
Propel drug discovery breakthroughs with RoukenBio
Difficulty doesn’t deter us; it inspires us. Give us a problem, and we’ll find your solution. Our versatile specialists embrace every obstacle as an opportunity to showcase our innovative thinking and problem-solving prowess.
Access our Capabilities brochure
