Immuno-oncology
Immuno-oncology is transforming cancer care by turning the body’s own immune system into a powerful weapon against tumours. Despite breakthrough responses, patients still face unresponsiveness, therapeutic resistance, or severe side effects. The future demands more effective therapeutic solutions tailored to each patient’s tumour profile which balance safety with optimal activity. At RoukenBio, our expert immunology team delivers advanced, bespoke tumour cell assays to accelerate your oncology research and therapeutic development. We turn complex challenges into innovative solutions to help you drive the next generation of cancer therapies.

Our immuno-oncology assays
At RoukenBio, we offer a specialised portfolio of advanced and customisable in vitro human immune cell-based assays specifically designed to model the tumour microenvironment (TME) and support every stage of your therapeutic development.
With extensive experience across a range of therapeutic modalities - including bispecific T cell engagers (BiTEs), antibody drug conjugates (ADCs) and CAR-T therapies - we integrate primary immune cells or engineered reporter cell lines with engineered or native tumour cell targets to create physiologically relevant models.
Our custom cell engineering capabilities enable us to identify or generate tumour cell lines expressing targets that are best suited to the needs of your study. These are seamlessly incorporated into bespoke immune cell assays, providing robust platforms for evaluating your therapeutic's mechanism of action and functional impact.
Available and customisable assay formats include:
- Immune cell-mediated killing assays
- T cell dependent cellular cytotoxicity (TDCC)
- Antibody-dependent cellular cytotoxicity (ADCC)
- Antibody-dependent cellular phagocytosis (ADCP)
- In vitro exhausted T cell assays
- T cell suppression assays
- CAR-T cell generation/characterisation
- Antigen-specific T cell assays
- Immune cell polarisation
- Mixed Lymphocyte Reaction (MLR)
- Cytokine production
Explore the diverse range of cell types and assays that RoukenBio offers focusing on immuno-oncology research.
Cells in immuno-oncology assays
Exhausted T cells
Macrophages - TAMs
Tregs
Exhausted T cells
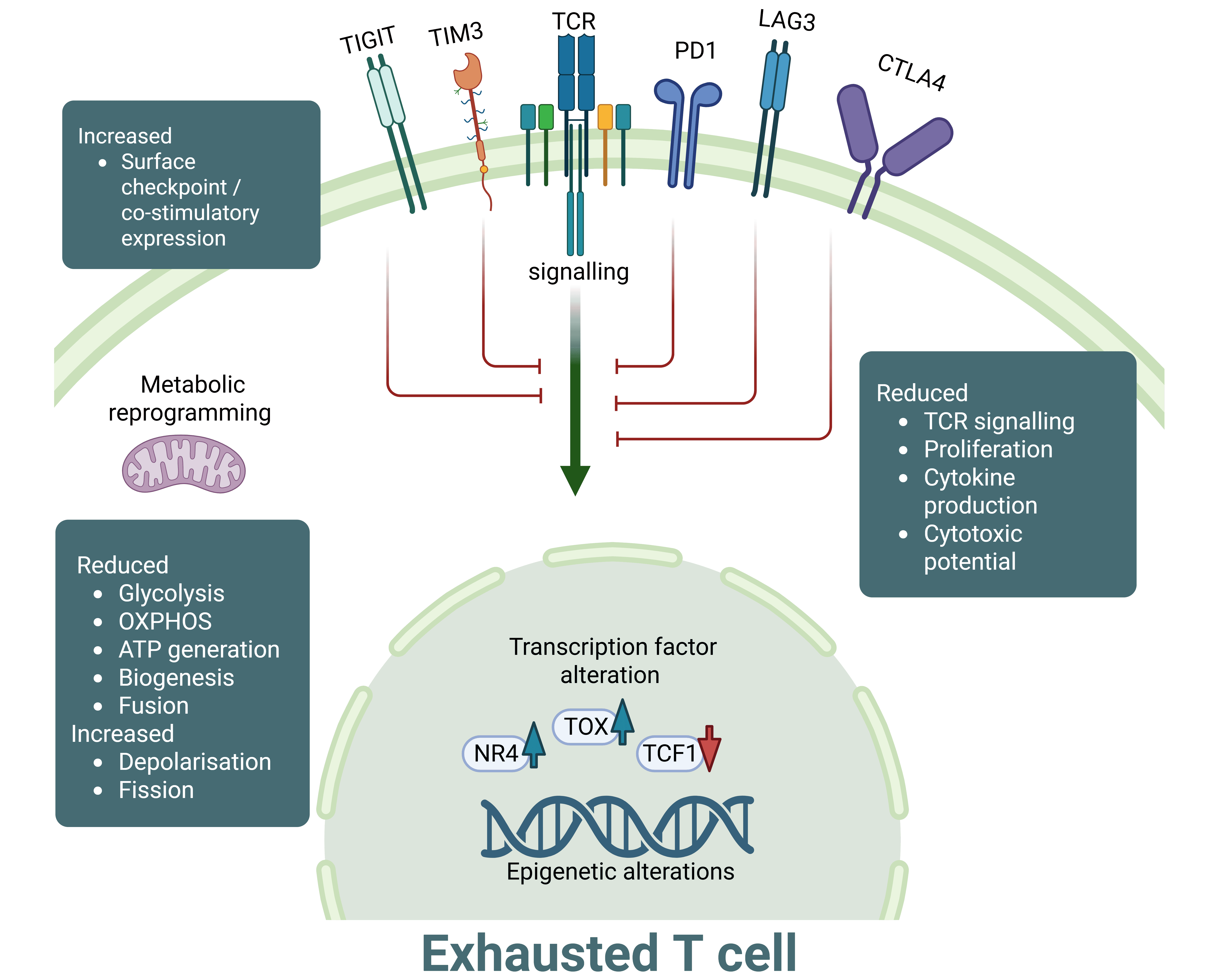
T cell exhaustion is a distinct differentiation state of T cells observed during chronic infections and cancer; in which chronic antigen exposure leads to T cell dysfunction. Exhausted T cells (TEX) are characterised by reduced proliferative responses to stimuli, loss of effector function failing to control tumour growth, high expression of inhibitory receptors such as PD-1, LAG-3 and TIM-3 and reduced cytokine secretion in comparison to their conventional, non-exhausted counterparts.
TEX undergo extensive transcriptional and epigenetic reprogramming, leading to upregulation of exhaustion-associated factors like TOX and display metabolic disruption due to nutrient-deprived and hypoxic conditions of the TME. These cells become addicted to antigen in what is described as ‘antigen-dependent maintenance’. When chronic antigenic stimulation is withdrawn, TEX remain dysfunctional, suggesting a commitment to the exhausted T cell state.
At RoukenBio, we have extensive expertise in T cell biology including T cell exhaustion. We were the first CRO to develop a human in vitro T cell exhaustion model that recapitulates the dysfunctional behaviour of T cells in the TME.
Exhausted T cells

T cell exhaustion is a distinct differentiation state of T cells observed during chronic infections and cancer; in which chronic antigen exposure leads to T cell dysfunction. Exhausted T cells (TEX) are characterised by reduced proliferative responses to stimuli, loss of effector function failing to control tumour growth, high expression of inhibitory receptors such as PD-1, LAG-3 and TIM-3 and reduced cytokine secretion in comparison to their conventional, non-exhausted counterparts.
TEX undergo extensive transcriptional and epigenetic reprogramming, leading to upregulation of exhaustion-associated factors like TOX and display metabolic disruption due to nutrient-deprived and hypoxic conditions of the TME. These cells become addicted to antigen in what is described as ‘antigen-dependent maintenance’. When chronic antigenic stimulation is withdrawn, TEX remain dysfunctional, suggesting a commitment to the exhausted T cell state.
At RoukenBio, we have extensive expertise in T cell biology including T cell exhaustion. We were the first CRO to develop a human in vitro T cell exhaustion model that recapitulates the dysfunctional behaviour of T cells in the TME.
Macrophages - TAMs
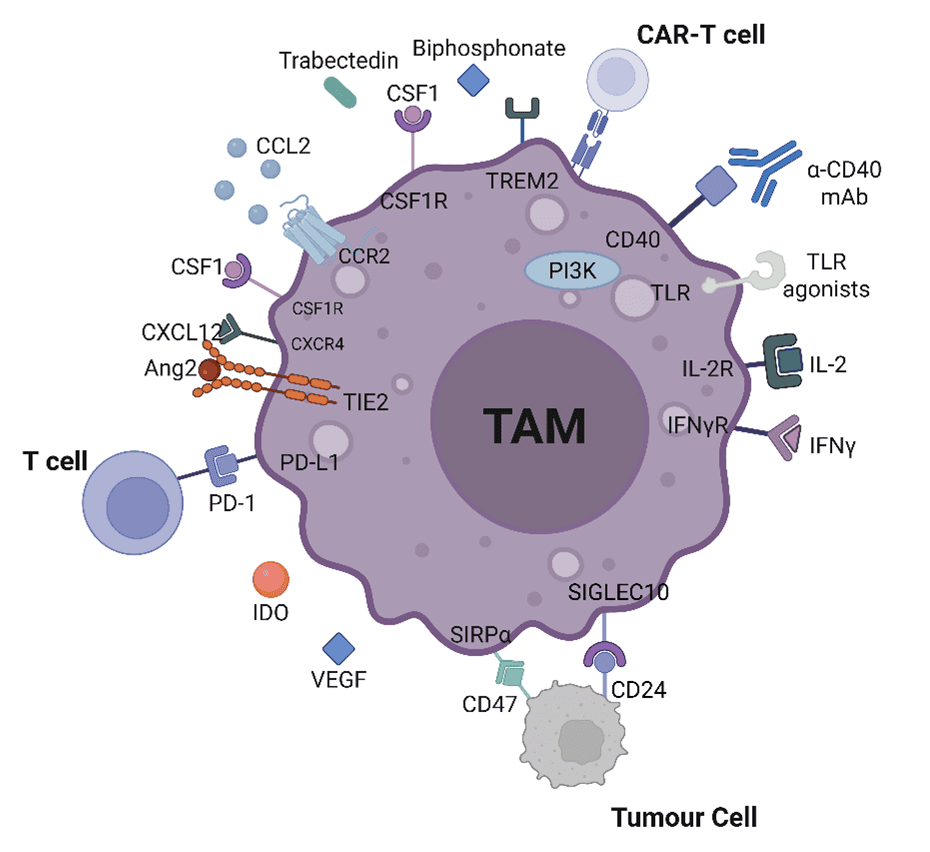
Tumour-associated macrophages (TAMs) are a dominant force within the immune landscape of solid tumours, playing a critical role in shaping the TME. Originating from bone-marrow monocytes or tissue-resident precursors, TAMs are remarkably adaptable, constantly reshaping their function in response to environmental cues. Macrophages are traditionally categorized into M1 (classically activated) and M2 (alternatively activated) subsets.
In the context of cancer, M1-like TAMs, triggered by type 1 cytokines such as IFN-γ, are associated with pro-inflammatory responses and tumouricidal activity. In contrast, cytokines like IL-4, IL-10, IL-13, TGF-β and PGE2, drive TAMs towards M2-like phenotypes that suppress anti-tumour immunity, support tumour growth, and contribute to remodelling of the TME. This impacts the success of certain therapeutics in treating the disease.
In most established tumours, TAMs adopt an M2-like phenotype, - marked by high levels of ARG1, CD163, and CD206 - and secrete immunosuppressive cytokines like IL-10 and TGF-β. These cells actively support tumour progression by promoting angiogenesis, remodelling tissue structure, and dampening anti-tumour immune responses via PD-L1, IDO, prostaglandins and the recruitment of regulatory T cells. TAM phenotypes vary widely across tumour types, and even within different lesions or stages of the same tumour. While the M1/M2 classification is a simplification, it remains a valuable framework for understanding macrophage function; especially when applied in the context of evaluating therapeutic candidates.
Key strategies for therapeutic targeting of macrophages in the TME include; CSF1(R) or CCL2/CCR2 inhibition resulting in TAM depletion or prevention of TAM recruitment, VEGF inhibition limiting their tumour-promoting activity, and using CD47/SIRPa inhibitors or TLR agonists to subvert their immunosuppressive function, aiming to re-educate M2-like TAMs to M1-like phenotypes.
At RoukenBio, we understand the complexity of TAM biology. Our advanced macrophage polarization, phagocytosis, and T cell suppression assays are designed to help you evaluate how your therapeutic candidates modulate TAM function, suppress tumour cell growth and drive anti-tumour immunity.
Tregs
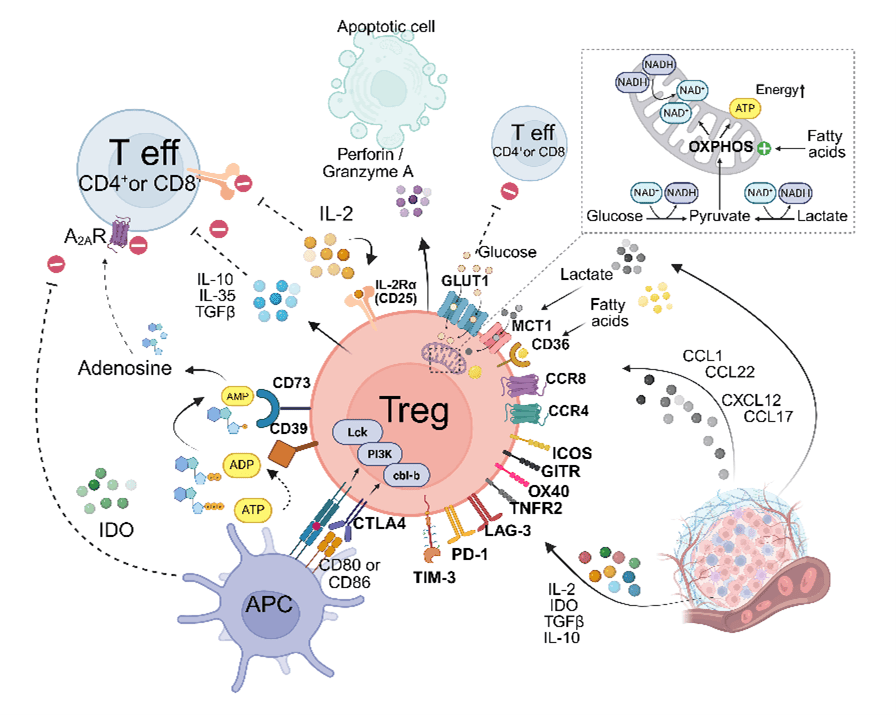
Regulatory T cells (Tregs), typically identified by the CD4⁺CD25highFOXP3high phenotype, represent a critical immunosuppressive component within the TME across a broad spectrum of cancers. Their enrichment in tumour tissues frequently correlates with poor cancer prognosis, highlighting their relevance as both biomarkers and therapeutic targets.
Tumour-infiltrating Tregs originate from diverse sources, including thymus-derived Tregs and peripherally induced Tregs derived from conventional T cells. In tumours, these cells exhibit a highly activated phenotype, marked by elevated expression of key immunoregulatory molecules such as CTLA-4, GITR, ICOS, OX40, and TIGIT, along with heightened levels of immune checkpoint receptors like PD-1, LAG-3, and TIM-3.
Functionally, Tregs suppress anti-tumour immune responses via multiple mechanisms: inhibition of effector T cell proliferation (IL-2 sequestration, ATP conversion, glucose deprivation), secretion of immunosuppressive cytokines (IL-10, TGF-β), and modulation of dendritic cell maturation and function (CD80/86 engagement via CTLA-4).
Therapeutic strategies aim to; deplete Treg (anti-CTLA-4), prevent their infiltration (CCR4 antagonists), or impair their immunosuppressive function in the TME (CD39/CD73 blockade) without compromising peripheral immune tolerance.
At RoukenBio, we offer comprehensive capabilities to evaluate how your therapeutic candidate influences Treg biology, providing in-depth analysis into the phenotype and functional activity of both natural and induced Treg populations.
Immuno-oncology assays and tools
Immune cell-based killing assays
Reversal of T cell exhaustion
T cell suppression assays
Immune cell-based killing assays
Immune cell-based killing assays are powerful tools for driving innovation in cancer immunotherapy, offering mechanistic insights and therapeutic evaluation of immune-mediated tumour cell destruction. By mimicking real-time tumour–immune cell interactions, these assays enable precise assessment of potency, specificity, and safety for cutting-edge modalities such as immune cell engagers and engineered cell therapies.
Beyond efficacy testing, they illuminate mechanisms of resistance and inform smarter therapeutic design. For researchers aiming to accelerate innovation in immuno-oncology, these assays are essential for translating breakthrough science into next-generation cancer treatments.
Our off-the-shelf and bespoke assay design allows us to test the cytotoxic potential of small molecules, biologics (BiTEs or ADCs) and cell-based therapies (CAR-T, CAR-NK or γδ T cells) against cancer cell lines or custom-built target expressing cells.
Available formats include:
T cell mediated:
- T cell dependent cellular cytotoxicity (TDCC): Assess ability of BiTEs to engage primary human T cells to kill tumour cells
- CAR-T cells: Determine cytotoxic potential of your CAR-T cell candidates
- TCR-engineered T cells: Assess therapeutic molecules in an antigen specific system
NK cell mediated:
- Antibody dependent cellular cytotoxicity (ADCC): Assess ability of antibodies to engage primary NK cells to kill tumour cells
- CAR-NK cells: Determine cytotoxic potential of your CAR-NK cell candidates
γδ T cell-mediated:
- Screen γδ T cell-engaging therapeutics using our ex vivo expanded γδ T cells (CD3+ Vγ9+Vδ2+)
Standard readouts include:
- Flow cytometry- or impedance-based target cytolysis
- Effector cell phenotyping, proliferation and activation via flow cytometry
- Cytokine analysis by ELISA or multiplex bead-based array
Immune cell-based killing assays
Immune cell-based killing assays are powerful tools for driving innovation in cancer immunotherapy, offering mechanistic insights and therapeutic evaluation of immune-mediated tumour cell destruction. By mimicking real-time tumour–immune cell interactions, these assays enable precise assessment of potency, specificity, and safety for cutting-edge modalities such as immune cell engagers and engineered cell therapies.
Beyond efficacy testing, they illuminate mechanisms of resistance and inform smarter therapeutic design. For researchers aiming to accelerate innovation in immuno-oncology, these assays are essential for translating breakthrough science into next-generation cancer treatments.
Our off-the-shelf and bespoke assay design allows us to test the cytotoxic potential of small molecules, biologics (BiTEs or ADCs) and cell-based therapies (CAR-T, CAR-NK or γδ T cells) against cancer cell lines or custom-built target expressing cells.
Available formats include:
T cell mediated:
- T cell dependent cellular cytotoxicity (TDCC): Assess ability of BiTEs to engage primary human T cells to kill tumour cells
- CAR-T cells: Determine cytotoxic potential of your CAR-T cell candidates
- TCR-engineered T cells: Assess therapeutic molecules in an antigen specific system
NK cell mediated:
- Antibody dependent cellular cytotoxicity (ADCC): Assess ability of antibodies to engage primary NK cells to kill tumour cells
- CAR-NK cells: Determine cytotoxic potential of your CAR-NK cell candidates
γδ T cell-mediated:
- Screen γδ T cell-engaging therapeutics using our ex vivo expanded γδ T cells (CD3+ Vγ9+Vδ2+)
Standard readouts include:
- Flow cytometry- or impedance-based target cytolysis
- Effector cell phenotyping, proliferation and activation via flow cytometry
- Cytokine analysis by ELISA or multiplex bead-based array
Reversal of T cell exhaustion
RoukenBio leverages extensive experience in T cell biology, having pioneered the development of a robust in vitro model of T cell exhaustion in-house. Using isolated pan CD3+ T cells that are repeatedly stimulated, our system drives cells into a hyporesponsive, dysfunctional state, characterized by upregulation of multiple immune checkpoint molecules and a marked reduction in IL-2 production.
Combining in vitro generated exhausted T cells with a functional assay such as a mixed lymphocyte reaction (MLR), this medium-throughput, flexible assay format is ideally suited for mechanism-of-action (MoA) studies of checkpoint inhibitors, co-stimulatory agents, T cell engagers, and modulators of T cell signalling, tailored to support both early discovery and translational research.
Functional Readouts Include:
- Cytokine secretion (e.g. IFNγ via ELISA or cytokine multiplex assays)
- Proliferation (Ki-67 expression by flow cytometry)
- Co-inhibitory and co-stimulatory marker profiling via flow cytometry
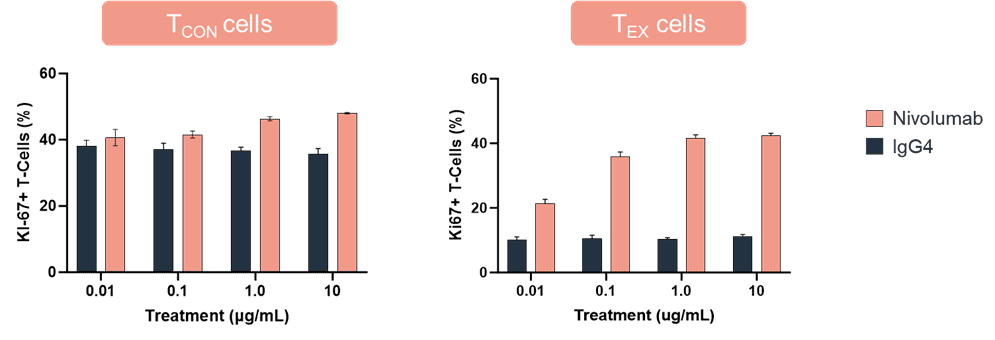
An example of data from an MLR functional readout is shown below. In this assay, proliferation as indicated by %Ki67 expression was compared in conventional T (TCON) cells and exhausted T (TEX) cells treated with nivolumab or an IgG4 control antibody.
T cell suppression assays
T cell fitness is often compromised by the complex and highly immunosuppressive nature of the TME. Within this dynamic ecosystem, diverse cellular populations - including regulatory T cells (Tregs) and tumour-associated macrophages (TAMs) - act through direct contact and soluble mediators to suppress T cell activation and proliferation, as well as limit their trafficking.
At RoukenBio, we specialise in T cell suppression assays using a variety of immunosuppressive cell types, including M2-polarised macrophages and both natural and induced Tregs. Our flexible platforms support evaluation across therapeutic modalities, helping you assess how your candidates modulate immune suppression.
We also offer test article integration during macrophage polarisation or Treg induction to evaluate effects on their differentiation.
Key readouts include:
- T cell proliferation and T cell phenotyping via flow cytometry
- Cytokine profiling through ELISA or multiplex array

Explore our collaboration with Miltenyi showcasing how Cbl-b antagonism reverses Treg-mediated T cell suppression.
Why choose RoukenBio for immuno-oncology assays?
Translational Focus
Off-the-shelf doesn’t cut it in I-O. Partner with experts in immuno-oncology to build custom assay systems using human primary cells in disease-relevant settings giving you the insight and edge to push innovation forward.
Rapid Turnaround. Relentless Quality.
We combine industry-leading turnaround times with a commitment to scientific excellence. Give us a problem, and we’ll find your solution.
10+ Years of Experience in Novel Cancer Immunotherapies
Grounded in experience and driven by curiosity, our brilliant minds work closely with our partners to deliver impactful science.
Propel your drug discovery forward with RoukenBio, the Immunology CRO redefined
Discover how our cutting-edge immuno-oncology assays can aid your therapeutic development. Explore our in vitro immune cell assay portfolio and capabilities.
Access our Capabilities brochure
