Immunogenicity and Safety Assessments
For protein based biotherapeutics, in vitro methodologies are crucial to assess immunogenic potential before clinical testing.

Immunogenicity, a key safety consideration, is influenced by factors such as treatment methods (e.g., route of administration, therapy duration and frequency), patient characteristics (e.g., immune status, genetic background, disease state) and drug properties (e.g., immunogenic peptide sequences, glycosylation patterns and impurities).
Immunogenic potential can be evaluated using in silico models, MHC-associated peptide proteomics (MAPPs) assay, and various in vitro immune cell assays. These methods identify T cell epitopes and are critical for optimising lead molecules.
DC Maturation Assays
T Cell Functional Assays
Cytokine Release Assay (CRA)
On-target, off-tumour assessment with IndEx-2
ADA ELISA
DC Maturation Assays
Dendritic cells (DCs) are key antigen-presenting cells. Assessing their maturation by monitoring cell surface marker expression (e.g., CD83, CD80, CD86, CD40), cytokine secretion (e.g., IL-1β, IL-6, IL-12, TNFα) or signalling pathway activation (e.g., Akt, Syk) via flow cytometry or ELISA provides insights into the immunogenic potential of biotherapeutics. These assays use monocyte-derived dendritic cells (moDCs), differentiated from PBMCs with GM-CSF and IL-4, to evaluate biotherapeutic-induced activation.
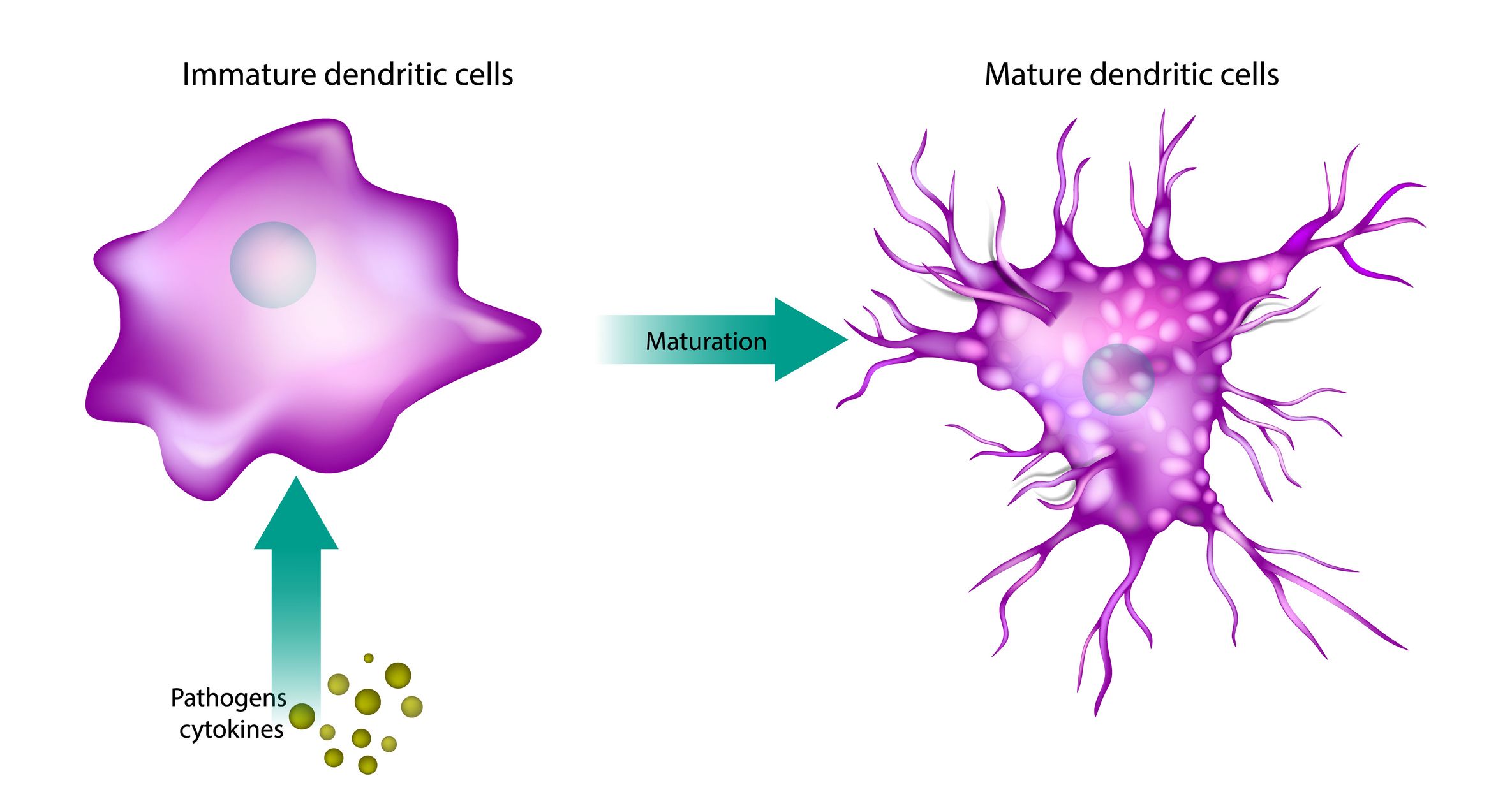
DC Maturation Assays
Dendritic cells (DCs) are key antigen-presenting cells. Assessing their maturation by monitoring cell surface marker expression (e.g., CD83, CD80, CD86, CD40), cytokine secretion (e.g., IL-1β, IL-6, IL-12, TNFα) or signalling pathway activation (e.g., Akt, Syk) via flow cytometry or ELISA provides insights into the immunogenic potential of biotherapeutics. These assays use monocyte-derived dendritic cells (moDCs), differentiated from PBMCs with GM-CSF and IL-4, to evaluate biotherapeutic-induced activation.

T Cell Functional Assays
T cell activation assays measure CD4+ T cell responses to therapeutic proteins, predicting immunogenic risk. Assay setups include PBMC-based or DC-T cell co-cultures, with readouts such as activation markers (CD154, CD134, CD137), cytokine secretion (IL-2, IFNγ, TNFα), and T cell proliferation (proliferation dyes, Ki-67 expression). Controls like KLH and benchmark drugs (e.g., bococizumab, ATR-107 and bevacizumab) ensure assay validity and comparative analysis.
Cytokine Release Assay (CRA)
In vitro CRAs predict cytokine release from immune cells exposed to biotherapeutics, aiding preclinical toxicology assessments. The basic principle of CRAs is incubation of immune cells with the biotherapeutic over a defined period followed by assessment of cytokine release usually with a multiplex approach (e.g., Luminex or MSD). Formats vary by immune cell source (whole blood or PBMCs) and biotherapeutic application (soluble or immobilised). Typically, a tiered approach is used, starting with multiple formats in limited donors, followed by a single format in many donors (8-20).
Important assay considerations include:
- Cell type employed: Blood vs PBMC; Fresh or Cryopreserved
- Assay format: Solid phase (immobilised) vs soluble therapeutic
- Number of donors
- Benchmark molecules
- MOA of the therapeutic
- Safety vs Functionality
On-target, off-tumour assessment with IndEx-2
The IndEx-2 system evaluates antigen density and activation thresholds to address on-target, off-tumour side effects in antibody-targeted therapies. This system controls the expression of one or two proteins over a dynamic range, linking antigen density to activation thresholds for cytolysis and cytokine release. This innovative approach supports screening, characterisation, and lead selection for mono- and dual-targeted therapies. At RoukenBio, we embrace change and drive innovation, exemplified by our trademarked IndEx-2 system.
ADA ELISA
Anti-Drug Antibody (ADA) ELISA is essential for detecting immune responses to biotherapeutics. This assay identifies and quantifies antibodies against therapeutic proteins, providing insights into immunogenicity thereby aiding in risk management and ensuring therapeutic efficacy and safety. Formats include screening assays to identify the presence of ADA, confirmatory assays to validate ADA specificity and neutralising assays to assess the inhibition of therapeutic protein activity by ADA.
Backed by brilliant minds
Learn how our IndEx-2 dual inducible cell line system can be used in combination with primary cell platforms to determine thresholds of activation. Discover the potential of 'on-target/off-tumour' effects for antibody-targeted therapeutic candidates.
WATCH THE WEBINAR
