Engineering immunity: How CAR-T cells are redefining cancer therapy development
CAR-T cell therapies are transforming cancer treatment, especially for blood cancers, with growing advances in solid tumours through innovative engineering and clinical strategies. These therapies harness genetically modified T cells to target tumour antigens and activate immune responses effectively.

|
August 18, 2025
|
7 min read
Chimeric Antigen Receptor (CAR)-T cell therapies have transformed blood cancer treatment and are making advances in solid tumours. This ground-breaking approach has achieved remarkable success in certain blood cancers, delivering high remission rates even in patients who failed standard therapies1,2. CAR-T cells have been called “living drugs” for their ability to persist and fight cancer inside the body. Their strength lies in precise targeting of tumour-associated antigens and activating the immune system at scale.
As research evolves, engineering breakthroughs are overcoming key challenges like antigen heterogeneity and immune suppression. In this post, we take a deeper look at how CAR-T cells work, their clinical impact in oncological indications, and the advanced technologies expanding their potential. We are also sharing two CAR-T case studies in the slide deck with you in case you’d like more details about how the potential of CAR-T cells can be harnessed for novel immunotherapy approaches.
What are CAR-T cells?
CAR-T cells are generated from T lymphocytes collected from patients, genetically engineered to express a CAR targeting at least one tumour antigen, then expanded and infused back to attack the patient cancer. This enables them to identify and eliminate cancer cells without reliance on major histocompatibility complex (MHC) presentation, a critical capability given that cancer cells frequently evade immune surveillance by downregulating MHC expression. The term "chimeric" in CAR refers to the fusion of an antibody-derived antigen-binding domain with T cell signalling regions, which serve to activate the T cell.
A typical CAR structure includes:
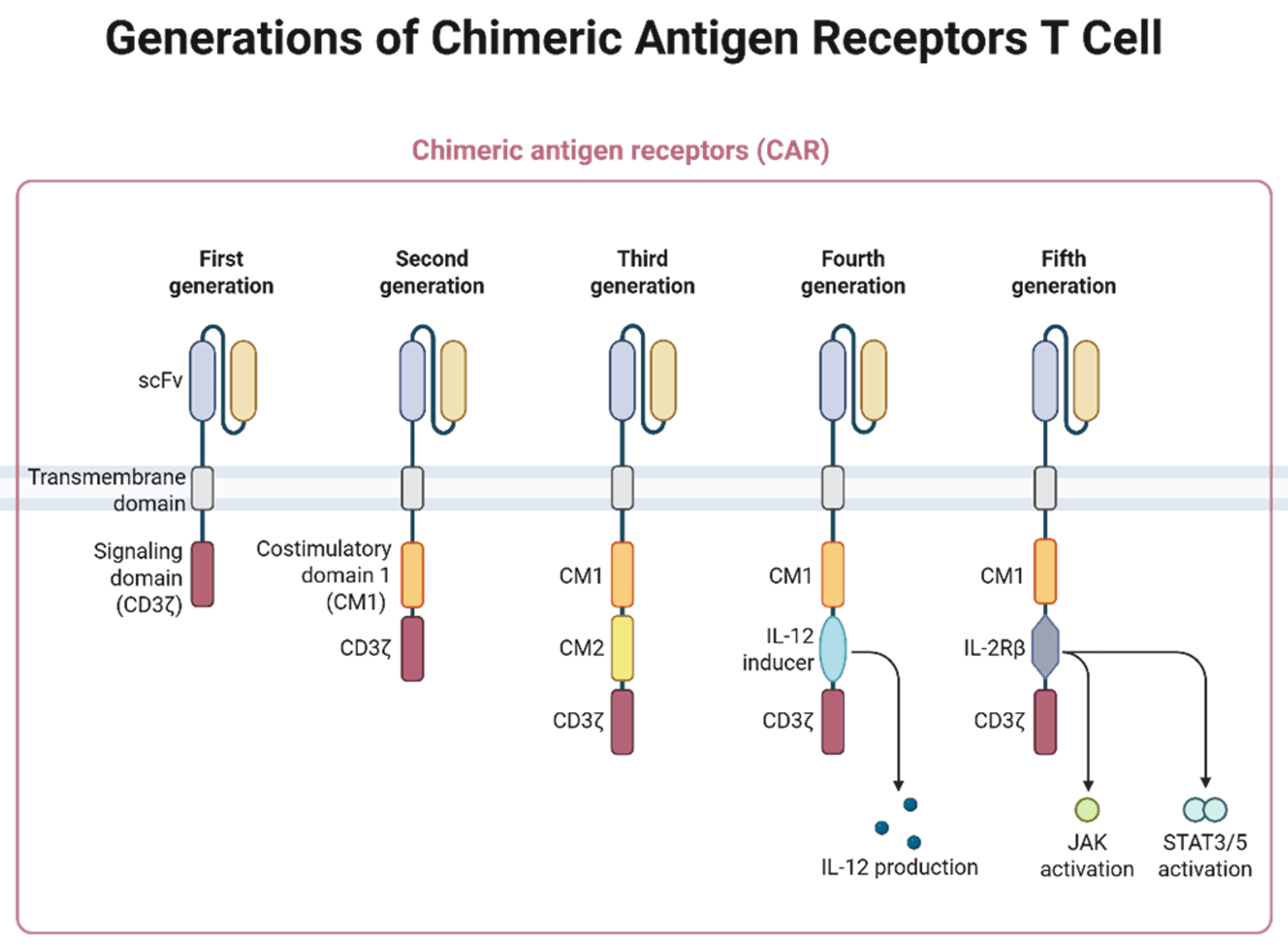
- An extracellular antigen-recognition domain (usually a single-chain variable fragment, scFv, derived from an antibody).
- A hinge (spacer) region.
- A transmembrane domain.
- An intracellular signalling region (CD3ζ and, for later generations, one or more costimulatory domains such as CD28 or 4‑1BB, inducers for intracellular production of the target cytokines or intracellular fragments of cytokine receptors).
At RoukenBio, our specialists design, engineer, produce and characterise CAR-T cells for targeted immune cell therapies utilising our in-house CAR-T capabilities. Alternatively, our molecular biologists can design CAR constructs to your custom specifications.
How CAR-T cells work: activation & killing
The way CAR-T cells carry out their function can be divided into two stages:
1. Recognition and activation
When the binding region of the CAR recognises its target, the receptor clusters and activates intracellular signalling domains like CD3ζ, which are phosphorylated at ITAM sites. This triggers downstream signalling (e.g. ZAP-70 activation) leading to T-cell activation, proliferation, and cytokine release, all essential for initiating a robust immune attack against tumour cells.
2. Tumour cell destruction
Once activated, CAR-T cells kill tumour cells via multiple mechanisms:
- Cytotoxic granules: They release perforin, which creates holes in cancer cell membranes, and granzymes, which induce apoptosis.
- Cytokine secretion: CAR-T cells produce cytokines (including IFN-γ and IL-2) that can reshape the tumour microenvironment (TME) and recruit additional immune cells to destruct the tumour cells.
Together, these processes ensure targeted and effective elimination of cancer cells, often with dramatic clinical outcomes. To make CAR-T therapies viable in more indications, especially solid tumours, researchers are developing strategies to overcome four major challenges: immunosuppressive TME, antigen specificity, resistance, and targeted delivery.
Challenges & advanced engineering in CAR-T Cell therapy
While CAR-T cell therapy has delivered remarkable results in blood cancers in recent years, its broader success in solid tumours, faces several biological and engineering hurdles. Researchers are actively developing CAR-T technologies to overcome these obstacles through innovative molecular designs and combination strategies. Let’s have a quick look at these challenges.
1. Immunosuppressive TME
One of the biggest challenges for CAR-T therapies in solid tumours is the immunosuppressive TME. Unlike blood cancers, solid tumours often present complex defence systems that actively inhibit T cell function. This includes the recruitment of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), as well as the secretion of immunosuppressive cytokines like TGF‑β and IL‑10. Tumour cells may also express checkpoint ligands such as PD‑L1, which dampen T-cell activation.
To counteract these, scientists are:
- Engineering CAR-T cells with dominant-negative checkpoint receptors, which block suppressive signals like PD-1/PD-L13.
- Designing armoured CAR-T cells secreting immune-boosting cytokines (like IL-12 or IL-18)4.
- Combining CAR-T therapy with checkpoint inhibitors, such as anti‑PD‑1 or anti‑PD‑L1 antibodies, to restore T cell activity5.
- Targeting suppressive immune cells directly, by designing CARs that recognise markers like CD123 on tumour-associated macrophages (TAMs), enabling dual-action cytotoxicity against both cancer and its immune shield6.
2. Tumour heterogeneity and specificity
Another major obstacle is tumour heterogeneity, more specifically the variation in antigen expression levels and density both within and between tumours. Furthermore, one major related challenge is the risk of on-target, off-tumour (OTOT) toxicity when healthy tissues express low levels of the target antigen. To navigate these, CARs are now being engineered with logic gating systems that act like molecular decision-makers:
- AND-gate / dual CARs: T cells activate only when two distinct tumour antigens are simultaneously detected, improving specificity7.
- Tandem CARs (TanCARs): These OR-gate receptors can trigger T cell activity upon detection of either of two tumour antigens, broadening recognition8.
- Universal CAR: Fifth-generation CARs use a "lock-key" system to separate antigen recognition from T cell signalling, enabling flexible and specific targeting of multiple antigens9.
- Inhibitory CARs (iCARs): These NOT-gate receptors deactivate T cell responses in the presence of antigens found on healthy tissue, reducing the risk of OTOT toxicity10.
3. Resistance and antigen loss
Tumours are adaptive and may escape immune pressure by shedding or mutating the target antigen. For example, BCMA, a key target in multiple myeloma, can be cleaved from the cell surface by γ-secretase, leading to antigen loss and therapy resistance11.
To address this, researchers are exploring combination therapies, such as pairing BCMA CAR-T cells with γ-secretase inhibitors, to increase antigen density on tumour cells and reduce the risk of relapse12.
4. Delivery to solid tumours
Finally, effective delivery of CAR T cells to solid tumours remains a major hurdle due to poor infiltration and physical barriers within the tumour mass. Innovative bioengineering approaches are being tested to enhance local delivery and retention:
- Microneedle patches13 and thin-film scaffolds14 are being developed to deliver CAR T cells directly into or around tumours, improving their ability to penetrate the tumour stroma and remain active at the site.
5. Other considerations
Safety and Side Effects: CAR-T therapy can trigger acute toxicities such as cytokine release syndrome (CRS), which ranges from mild to life-threatening and may require intensive care. Early trials saw high rates of severe CRS, but improved protocols and early use of tocilizumab have reduced incidence to about 10–20% in some cases15. Neurotoxicity (ICANS), including confusion or seizures, occurs in 20–50% of patients but is often manageable with monitoring and steroids16. Most patients recover without long-term effects, and treatment-related mortality is under 3% at experienced centres. Due to these risks, CAR-T is restricted to hospitals with ICU support and requires close patient monitoring15,16.
Manufacturing, Cost, and Access: CAR-T therapy requires complex, individualised production that takes 1–2 weeks per patient, leading to high costs ($375,000–$600,000+) and limited availability17. Insurance may help, but affordability and access are major issues, especially outside wealthy countries and specialised centres. Travel and manufacturing delays can affect outcomes. Regulators face challenges ensuring quality and timely approvals, while ethical concerns about fair access, ongoing monitoring, and informed consent persist. Streamlining production and reducing costs are essential for broader use18.
6. Future Directions and Emerging Developments
The next steps for CAR-T therapies aim to expand their use, enhance safety, and lower costs. A major innovation is developing allogeneic, "off-the-shelf" CAR-T cells from donors, modified with gene-editing tools like TALEN or CRISPR to minimise immune issues19. These treatments are in early trials and could offer faster, more affordable access. Another exciting strategy under investigation is in vivo CAR-T generation, where vectors delivered directly to patients reprogram T cells inside the body, potentially streamlining treatment further, though this method remains largely experimental20.
New cell therapy platforms are emerging, such as CAR-NK cells, which use engineered Natural Killer cells that can kill tumours with potentially fewer side effects and allogeneic compatibility. Early trials in leukaemia and lymphoma show promising results for CAR-NK21, and other approaches like CAR-macrophages and TCR-engineered T cells are being studied22. The first approval of TIL therapy for melanoma in 202223 has increased optimism for adapting CAR-T to solid tumours.
As CAR-T therapy becomes more widely adopted, clinicians are testing its use earlier in treatment, including replacing second transplants or serving as a frontline option for high-risk cases. Studies like ZUMA-12 have shown high response rates when CAR-T is used initially in aggressive cancers24. Efforts are ongoing to make CAR-T safer and more accessible, including outpatient administration and improved toxicity management.
Experts acknowledge that CAR-T is not a panacea, and continuous research is critical to tackle its current limitations. Key areas of active research include improving CAR-T cell persistence and exhaustion, identifying better tumour antigens (especially for solid cancers), enhancing manufacturing efficiency, and managing immune side effects with greater precision.
CAR-T clinical highlights & recent advances
In June 2025, Gilead and University of Pennsylvania reported promising results for a novel CAR-T cell therapy targeting recurrent glioblastoma25. Tumours shrank in 62% of evaluable patients, with manageable neurotoxicity and some patients achieving stability beyond 6 months. The CAR targeted both EGFR and IL‑13Rα2.
At ASCO 2025, a randomised trial in advanced gastric and gastro‑oesophageal junction cancers showed CAR-T cell therapy extended survival by around 40%26, a milestone road‑map for solid tumour application. The CAR targeted CLDN18.2 positive gastric or gastroesophageal junction cancer.
- As interest in in vivo CAR-T technology increases, two acquisitions in the field have been reported in 2025: AstraZeneca acquired EsoBiotec27, while Abbvie acquired Capstan Therapeutics28.
The science behind CAR-T cell therapy in summary
CAR-T cell therapy is transforming cancer treatment, showing strong results in blood cancers and progress with solid tumours. Advances in engineering are addressing challenges like tumour heterogeneity and delivery. While there are safety, cost, and access issues, ongoing innovations (including allogeneic and in vivo platforms) are making these therapies safer and more available. Recent clinical and technological breakthroughs suggest CAR-T could soon become a standard option for more cancers, supported by continued research and collaboration.
Join our community of curious minds on LinkedIn
🗓️ Stay informed with our monthly scientific newsletter, published on LinkedIn on the last Wednesday of each month.
These editions bring you the latest in drug development breakthroughs, industry trends, and expert insights from the brilliant minds at RoukenBio.
Subscribe today on LinkedInAdvance your CAR T therapy with RoukenBio
Are you developing the next breakthrough in CAR T cell therapy? Discover how our expertise can accelerate your journey. Explore two real-world CAR T case studies and learn how our platforms and capabilities are designed to support cutting-edge cell therapy development.
Download our CAR T technical slides

