Allergy
At RoukenBio, we specialise in advanced bioassays that use a range of cell types central to allergy mechanisms. Our assays focus on effector cells like mast cells, basophils, and eosinophils, as well as the Th2 and B cells that play a crucial role in allergic immune responses.

Our allergy assays
Allergies, or allergic diseases, encompass a variety of conditions where the immune system mounts inappropriate responses to normally harmless environmental substances such as pollen or foodstuff. These allergic responses are typically mediated by immunoglobulin E (IgE) bound to tissue mast cells and blood basophils triggering release of immune mediators upon exposure to these environmental substances (allergens). Affected individuals experience a range of symptoms, from mild reactions such as sneezing, itching, and coughing to severe, life-threatening responses as seen in anaphylaxis. Current therapies broadly suppress the immune response, but novel treatments targeting the underlying mechanisms of allergies offer the potential for more effective solutions.
At RoukenBio, we specialise in bioassays employing a variety of cell types relevant to key immune processes underlying allergy. They range from direct effector cells such as mast cells, basophils and eosinophils, to the Th2 and B cells underpinning allergic adaptive immune responses. Our advanced immunological & bioanalytical techniques help identify and analyse small molecule compounds and antibodies, which are essential in regulating biological processes. By leveraging cutting-edge technology and scientific expertise, we aim to uncover new therapeutic targets and pave the way for innovative treatments.
Explore the diverse range of cell types and assays that RoukenBio offers focusing on allergy research.
Cells in allergy
Mast cells
Eosinophils
Th2 cells
Mast cells
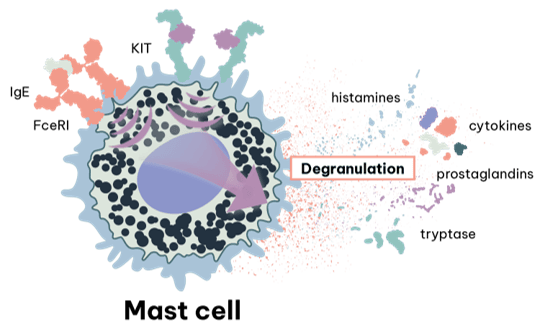
A central event in acute allergic reactions is the triggering of mast cells, granulated-tissue-resident innate cells found in peripheral tissues such as skin, airways and gastrointestinal tract. Antigen-specific activation of these mast cells occurs when surface bound IgE is crosslinked by binding of an allergen, driving activation of the mast cell via the high affinity IgE receptor (FεRI). Triggering in this way results in mast cell release of a wide range of proinflammatory and vasoactive mediators derived from preformed granules, arachidonic acid metabolites and as cytokines. Key examples include histamine, proteases (e.g., tryptase, chymase) and enzymes (e.g., betahexoaminidase); prostaglandins and leukotrienes (e.g., PGD2, LTC4 respectively); and cytokines (e.g., IL-1β, IL-6). This ability to release a wide range of mediators, makes them key players in modulating the immune response and therefore mast cells and their products are attractive therapeutic targets.
A significant barrier to studying human mast cell activity arises from their tissue resident nature, making acquiring sufficient cell numbers for assay use challenging. RoukenBio offers in vitro mast cell differentiation as a valuable tool for use in functional assays for the screening of candidate therapeutics, as well as custom cell lines to monitor triggering of FεRI.
Mast cells

A central event in acute allergic reactions is the triggering of mast cells, granulated-tissue-resident innate cells found in peripheral tissues such as skin, airways and gastrointestinal tract. Antigen-specific activation of these mast cells occurs when surface bound IgE is crosslinked by binding of an allergen, driving activation of the mast cell via the high affinity IgE receptor (FεRI). Triggering in this way results in mast cell release of a wide range of proinflammatory and vasoactive mediators derived from preformed granules, arachidonic acid metabolites and as cytokines. Key examples include histamine, proteases (e.g., tryptase, chymase) and enzymes (e.g., betahexoaminidase); prostaglandins and leukotrienes (e.g., PGD2, LTC4 respectively); and cytokines (e.g., IL-1β, IL-6). This ability to release a wide range of mediators, makes them key players in modulating the immune response and therefore mast cells and their products are attractive therapeutic targets.
A significant barrier to studying human mast cell activity arises from their tissue resident nature, making acquiring sufficient cell numbers for assay use challenging. RoukenBio offers in vitro mast cell differentiation as a valuable tool for use in functional assays for the screening of candidate therapeutics, as well as custom cell lines to monitor triggering of FεRI.
Eosinophils
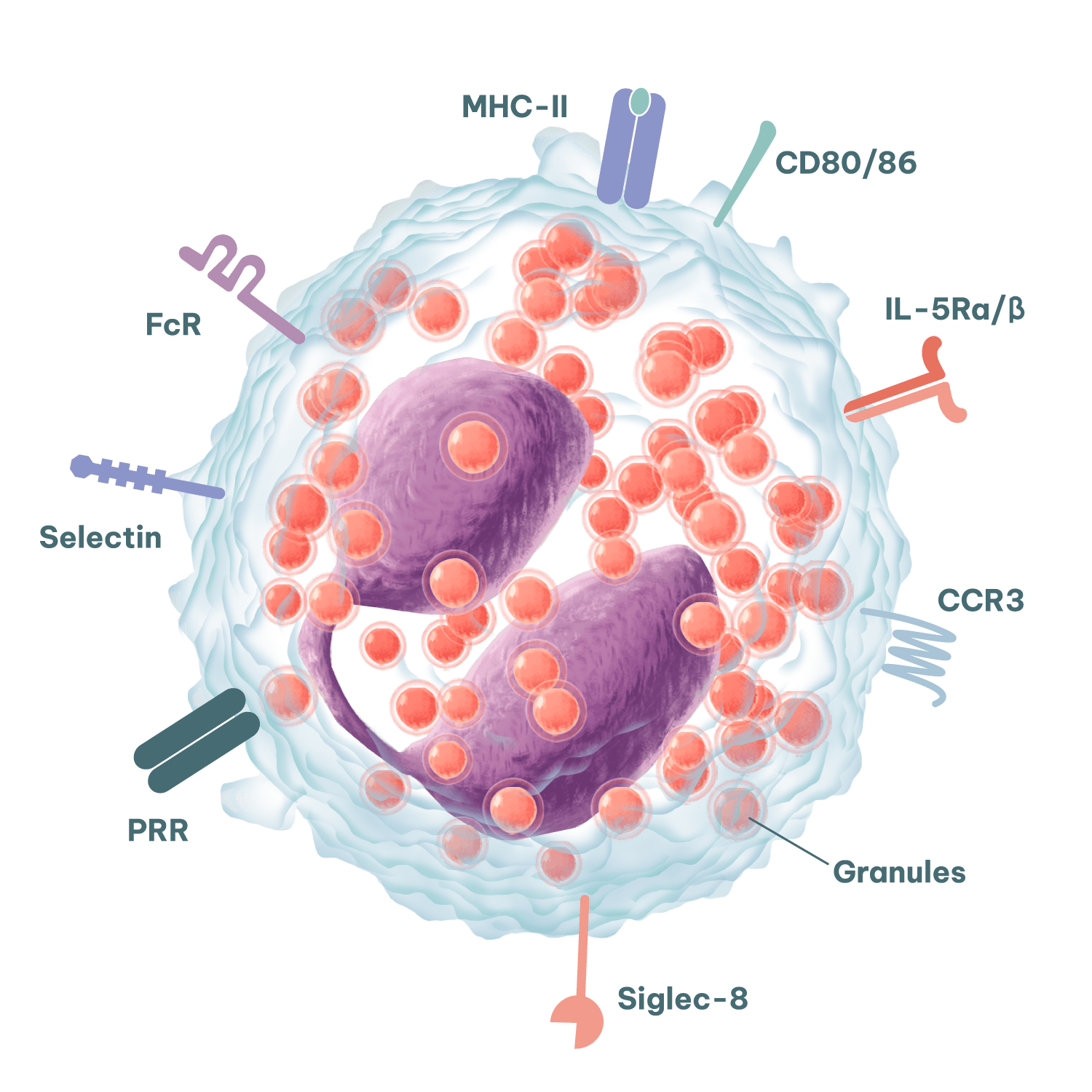
Recruitment of eosinophils to sites of allergic inflammation is associated with excessive tissue damage. Upon activation, eosinophils release a host of cytotoxic proteins including major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN) and eosinophil peroxidase (EPO). In addition, eosinophils can release a host of immunomodulatory cytokines and chemokines to shape adaptive immune responses.
Th2 cells
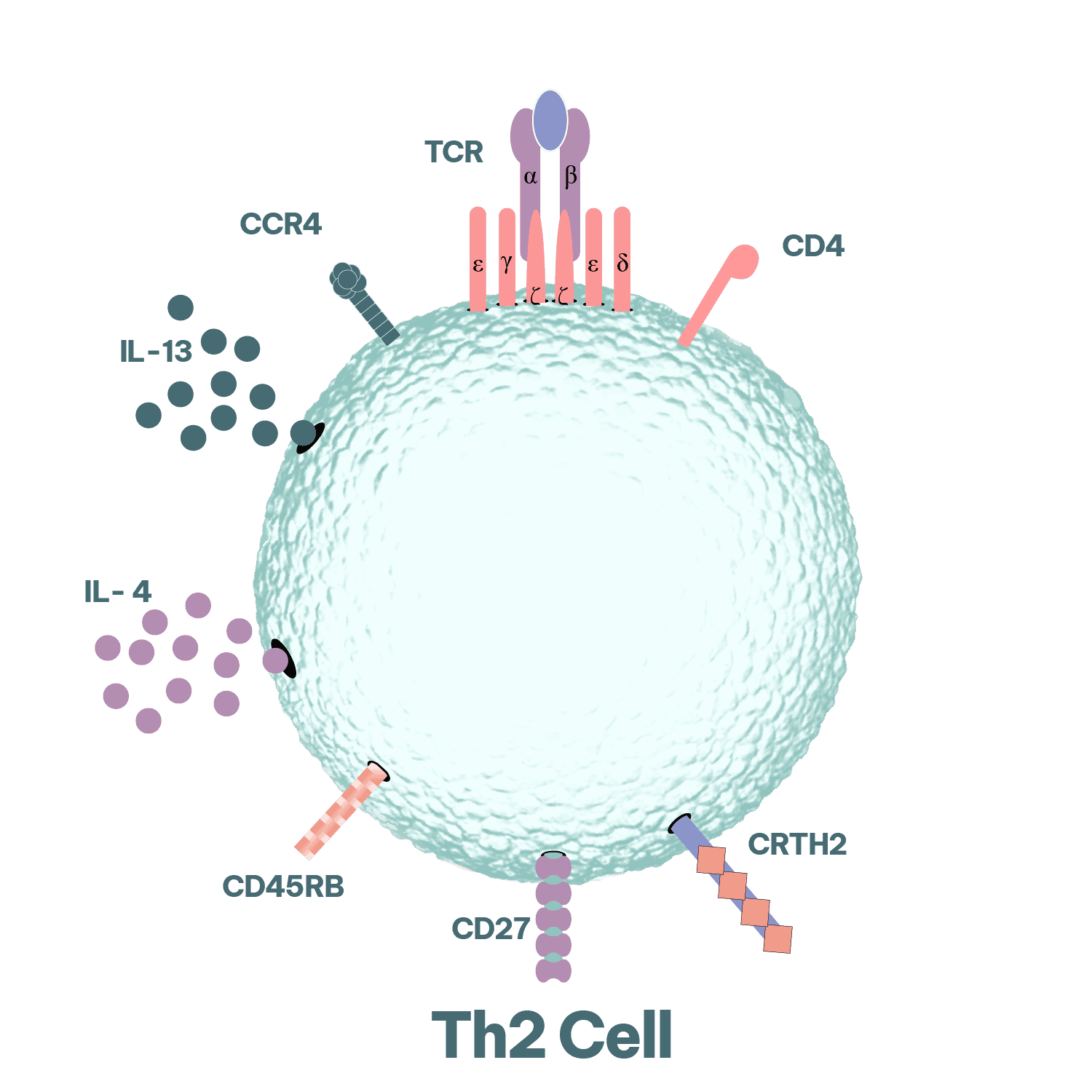
CD4 T helper (Th cells) choreograph adaptive immune responses. Th2 cells are associated with helminth infections, however their activity also underlies the inappropriate immune responses seen in allergy. Their activation and secretion of typical Th2 cytokines (IL-4, IL-5, IL-9 and IL-13) drive B cell proliferation and immunoglobulin class-switching to IgE, eosinophilia and mastocytosis, goblet cell hyperplasia, alternative macrophage activation (M2 polarisation) and smooth muscle contraction in type 2 immune responses. Discover more on our T cell assays.
Allergy assays and tools
Primary mast cell differentiation
Primary eosinophil isolation
Functional allergy assays
Reporter cell lines
Primary mast cell differentiation
h-Differentiation (CD34+): Human CD34+ cells isolated from healthy donor blood and cultured for up to 8 weeks in the presence of recombinant hIL-3, hSCF and hIL-6. Immunophenotyping of the enriched population is performed by flow cytometry for classical surface differentiation biomarkers (e.g., c-Kit/CD117, FεRI) or activation biomarkers (e.g., CD63) upon in vitro activation.
Primary mast cell differentiation
h-Differentiation (CD34+): Human CD34+ cells isolated from healthy donor blood and cultured for up to 8 weeks in the presence of recombinant hIL-3, hSCF and hIL-6. Immunophenotyping of the enriched population is performed by flow cytometry for classical surface differentiation biomarkers (e.g., c-Kit/CD117, FεRI) or activation biomarkers (e.g., CD63) upon in vitro activation.
Primary eosinophil isolation
Freshly isolated from whole blood: Our on-site healthy blood donors allow ready access to human eosinophils isolated directly from freshly drawn blood. The highly enriched population can be immunophenotyped by flow cytometry for a variety of classical biomarkers (e.g. CCR3, IL-5Ra, CD49d, CD15, Siglec-8) and used in custom bioassays.
Functional allergy assays
Mast cell degranulation assay: Mature mast cells can be sensitized with IgE and mast cell activation/degranulation achieved in an antigen driven manner or by employing anti-IgE-antibody. Assay readouts can include measurement of soluble mediators (e.g., tryptase, histamine) by MSD or ELISA or surface biomarkers (e.g., CD63) by flow cytometry.
Reporter cell lines
- Human FεRI-NFAT reporter cell line: We have successfully engineered the RBL-H3 rat mast cell line to express human FcεR1 and luciferase reporter gene. Combined with our IgE sensitisation and crosslinking protocols, this represents a fast, high throughput option for screening candidate molecules for their impact on FεRI triggering.
- IL-4/IL-13 reporter cell line: Assess the direct impact of your molecule on IL-4 and IL-13 induced signal transduction using our luciferase reporter cell line. IL-4 and IL-13 regulate many aspects of allergic inflammation, driving key events such as Th2 differentiation, immunoglobulin class switch to IgG1 and IgE, and drive alternative macrophage activation.
- Human TSLPR-IL-7R reporter cell line: Known to promote type 2 responses as seen in allergic disease, TSLP is an epithelial derived cytokine promoting Th2 cell polarisation and stimulating dendritic cells, basophils, mast cells, ILC2s and eosinophils. In addition to being approved target in severe asthma, TSLP is an attractive therapeutic target in multiple allergic cascades. Test your TSLP targeting molecules using our engineered human TSLPR and IL-7R expressing luciferase reporter cell line.
This list is not exhaustive; our specialist team can develop the best assay to answer your questions. Give us a problem, and we’ll find your solution.
Why choose RoukenBio for allergy assays?
Allergy assays are transforming therapeutic development
From the primary mast cell differentiation to functional assays, our in-house experts and novel solutions are here to guide you through the drug development pipeline. Our allergy assays produce quality data to help you progress with your leads.
Expertise in primary biology assays
We leverage our in-depth knowledge of immune cell function to provide reliable and reproducible results.
Customizable assay platforms
Tailored solutions to meet specific research needs in allergy and IgE-targeted therapy development.
State-of-the-art technologies
Access to advanced flow cytometry, functional bioassays, and reporter-based systems.
Partner with us
Let us help accelerate your allergy research and therapeutic development. Contact us today to discuss how our bioassay expertise can support your next breakthrough in allergy treatment.
Access our Capabilities brochure
