Development of an SPR-Based Assay for Detection of Pre-Existing Anti-Drug Antibodies in Treatment-Naïve Human Serum
Anti-drug antibodies (ADAs) can derail a drug’s success before it ever reaches patients. In this study, we built a sensitive SPR-based assay to detect pre-existing ADAs in treatment-naïve serum helping drug developers make smarter, safer choices early on. From chip selection to control validation, here’s how we tackled the challenge.

|
November 4, 2025
|
5 min read
Anti-drug antibodies (ADAs) can impact drug safety, efficacy, and pharmacokinetics. Detecting pre-existing ADAs is critical in early stages of drug development to guide lead candidate selection and reduce clinical risk. This study aimed to develop an SPR-based assay to detect ADAs against a T-cell engager (TCE) in treatment-naïve human serum. As an initial step, sensor chip surfaces with varying dextran densities (CM5, CM3, and C1) were evaluated. The effect of a non-specific binding (NSB) reducing reagent was also investigated to minimise background signal. Following selection of a positive control, sensitivity and specificity were assessed to confirm assay suitability before sample testing.

Anti-drug antibody (ADA) assay format
The T Cell Engager (TCE) was immobilised using amine coupling chemistry on a variety of chip surfaces. This approach results in attachment of the ligands to the surface in a random orientation, increasing the likelihood of epitopes being available for binding to ADAs in the serum. The naïve serum was obtained from healthy donors rather as opposed to target disease population.
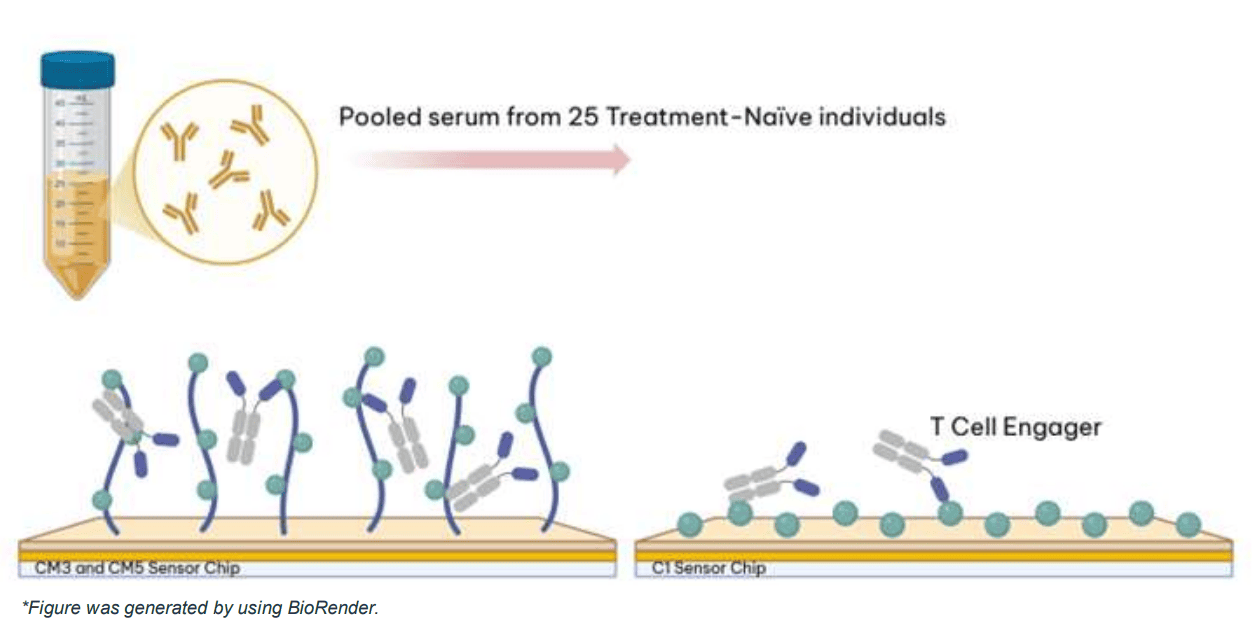
Chip selection and suitability
The purpose of this development run was to assess suitability of three different chip surfaces with varying levels of dextran (CM5, CM3, and C1) for the assessment of human serum samples. Human serum was flowed over a ligand-free surface in the presence and absence of NSB Reducer (Cytiva). Non-specific binding was highest on the C1 chip (flat carboxylation), followed by CM3 (short dextran), and CM5 (long dextran). The C1 sensor chip is less hydrophilic than the CM3 and CM5 chips, which likely contributes to increased non-specific binding when flowing serum over as analyte. Additionally, the NSB reducer (which contains dextran) effectively reduced the background response from 7 to 44%, depending on the sensor surface used.
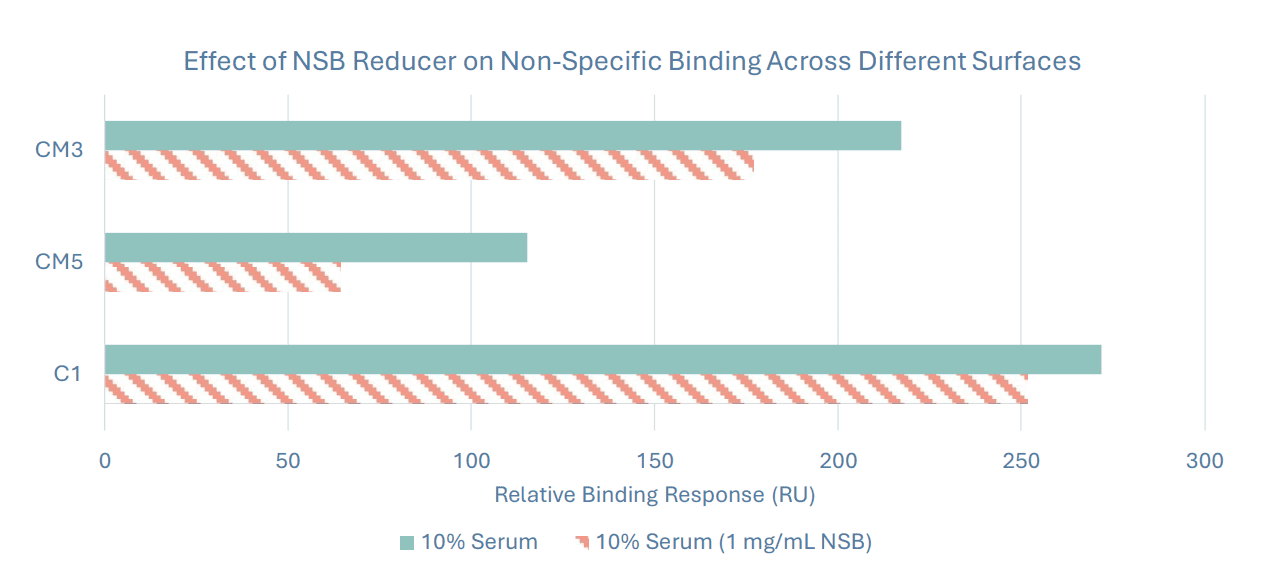
The following chip type and serum combination resulted in the lowest amount of non-specific surface binding and was used in subsequent development stages:
• Sensor chip - CM5
• Serum Dilution Buffer - HBS-EP+ containing 1 mg/mL NSB reducer
Positive control selection
Idiotypic controls are commonly used in ADA assays, but none were available for this TCE. To identify a suitable surrogate positive control, three antibodies were evaluated:

Binding to immobilised TCE was assessed in buffer (HBS-EP⁺) and 10% serum. In buffer, all showed concentration-dependent binding. In serum, both anti-Fc antibodies lost detectable binding, as expected. This was attributed to saturation by endogenous human IgG (~10 g/L). In contrast, the mAb anti-variable domain maintained a dose-dependent response in both diluents, with slow dissociation and complete regeneration, making it the ideal candidate to spike the serum in subsequent stages.
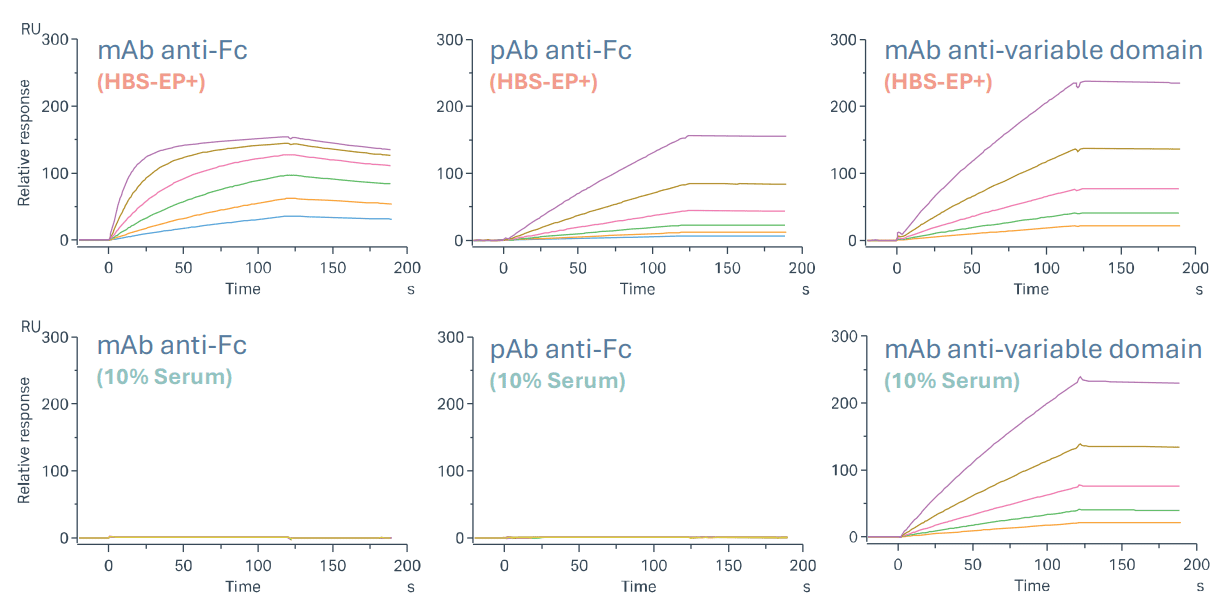
Assay sensitivity and specificity
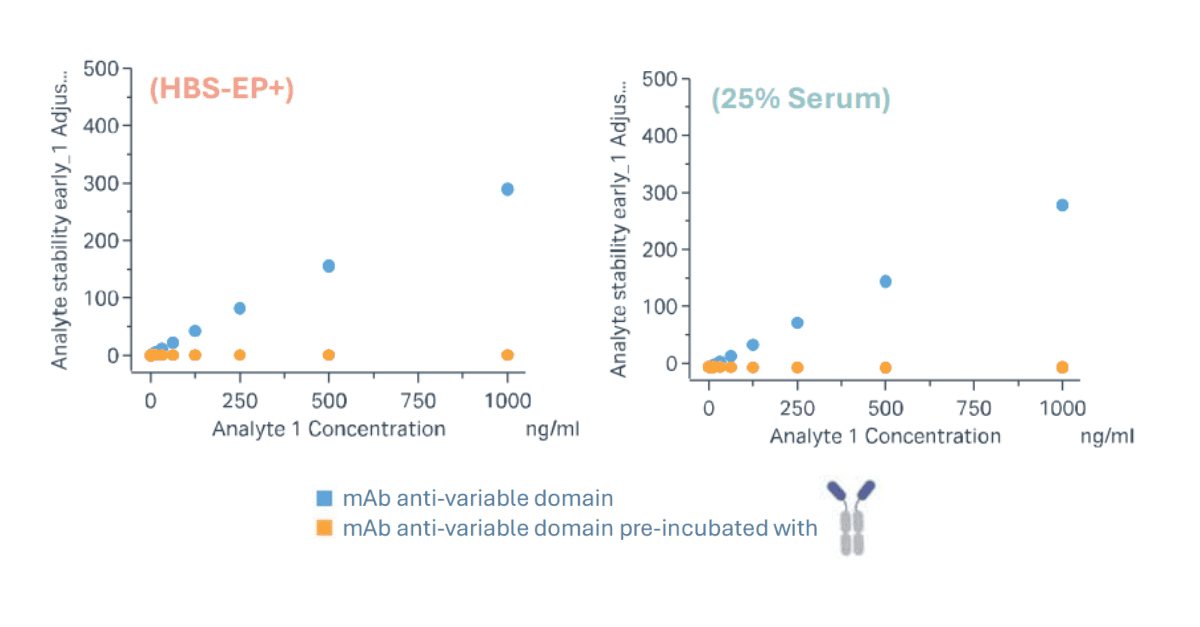
• Minimal Required Dilution (MRD)
A serum titration spiked with the positive control antibody was performed to determine the MRD. Serum samples were tested at dilutions of 1:4, 1:10, and 1:20. Based on the assay signal, an MRD of 1:4 was selected as it provided the optimal balance between minimising matrix interference and preserving assay sensitivity.
• Sensitivity
The lowest positive control concentration yielding a binding response >10 RU was 125 ng/mL at a 1:4 serum dilution (25% serum). This corresponds to an estimated assay sensitivity of 500 ng/mL in neat serum.
• Specificity
Specificity was demonstrated by pre-incubating the positive control with a saturating concentration of TCE (approximately a 6:1 molar ratio). This pre-treatment abolished the binding signal, confirming that the assay response is specific to the TCE-positive control interaction.
Summary
The objective of this study was to develop an early-phase ADA binding assay to detect pre-existing antibodies in treatment-naïve human serum to support lead candidate selection. A CM5 sensor chip combined with an NSB reducing reagent minimised serum non-specific binding and enabled accurate background subtraction using the reference flow cell. In the absence of an idiotypic control, an anti-variable domain mAb was identified as a surrogate. The assay showed suitable performance, with sensitivity of 500 ng/mL in neat serum and specificity confirmed by loss of response after pre-incubation with the TCE. Future work using individual serum samples will determine the assay cut-point prior to testing
Special thanks to Sylwia Marshall from LabGenius Therapeutics who helped in the creation of our collaborative research poster.
Figures were created by using BioRender.
Join our community of curious minds on LinkedIn
🗓️ Stay informed with our monthly scientific newsletter, published on LinkedIn on the last Wednesday of each month.
These editions bring you the latest in drug development breakthroughs, industry trends, and expert insights from the brilliant minds at RoukenBio.
Subscribe today on LinkedInDiscover our custom SPR assays
Download our SPR technical presentation to learn more about all our SPR capabilities and chat to our experts to see how we could collaborate and enhance your drug discovery journey.
Access the technical presentation


