Precision Kinetics: Leveraging SPR and scIC for Drug-Target Binding Analysis
Surface Plasmon Resonance (SPR) and Single Cell Interaction Cytometry (scIC) are essential tools for drug development kinetics. But how do they work, and which is more effective?

|
November 18, 2024
|
4 min read
Understanding how drugs bind to their biological targets is a cornerstone of effective therapeutic development. For insights into efficacy, specificity, and safety, an in-depth kinetic profile of these interactions is essential. Techniques like Surface Plasmon Resonance (SPR) and Single Cell Interaction Cytometry (scIC) provide drug developers with powerful, high-resolution tools for analysing binding interactions across biologics, small molecules, and cellular therapies. Here’s a look at the unique advantages of each technique.
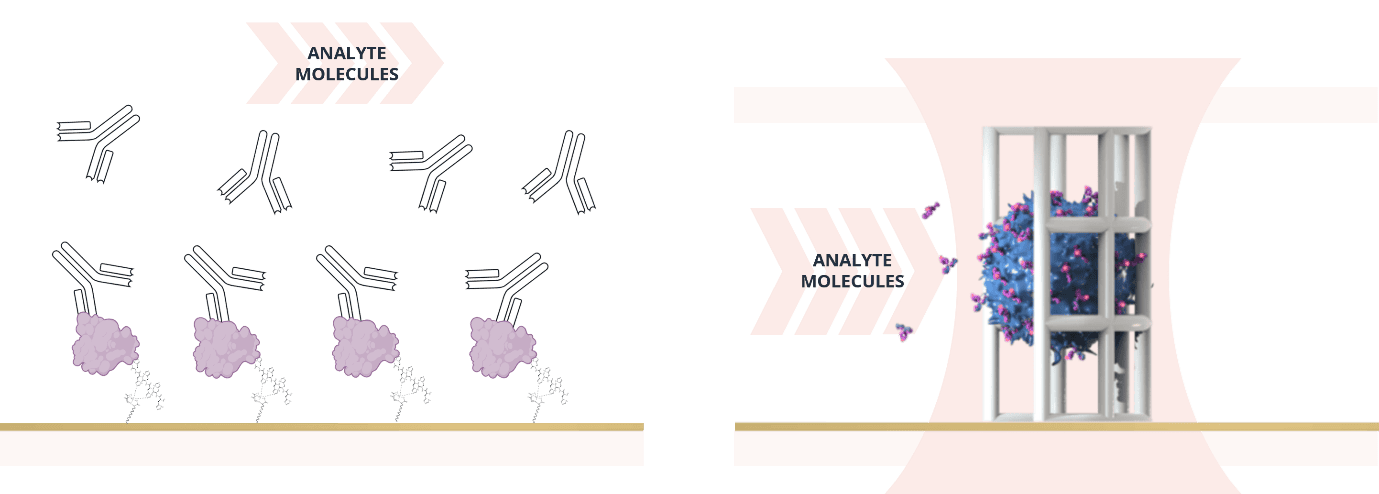
SPR (left) vs scIC (right) - powerful, high-resolution tools for the kinetic analysis of binding interactions.
What is SPR?
SPR is a label-free, real-time technique that monitors biomolecular interactions by detecting refractive index changes on a sensor surface when binding occurs. It’s a go-to technology for measuring key binding parameters - association rates (ka), dissociation rates (kd) and affinity constants (KD) - without the need for labels, which helps preserve the native interaction.
SPR's unique benefits
- Label-free detection: SPR provides a direct readout of interactions without the interference of labels, preserving native binding dynamics and enhancing data relevance.
- High sensitivity and precision: SPR’s ability to detect small refractive index changes enables precise measurements of binding kinetics, including low-affinity interactions with challenging targets like membrane proteins.
- Detailed kinetic profiles (ka, kd, KD): SPR provides complete kinetic profiles, offering insights into the stability and longevity of therapeutic binding, which is key for understanding therapeutic efficacy and half-life.
- Broad sample compatibility: SPR works with various biomolecular formats—proteins, peptides, DNA/RNA, and small molecules—making it versatile across different therapeutic modalities.
- High-throughput screening: Advanced SPR instruments like the Biacore 8K allow high-throughput screening, enabling rapid data collection and accelerating drug discovery timelines.
The Biacore 8K.
What is scIC?
scIC, facilitated by the heliXcyto biosensor, is a next-generation, chip-based technology that examines drug binding kinetics at the single-cell level. This approach provides real-time data on how therapeutic molecules interact with cell targets directly within their native cellular environment, capturing ka, kd, and KD with precision.
Unique benefits of scIC
- Native cellular context: scIC measures binding on the cell surface, preserving the target’s natural structure, density, and interactions. This is crucial for targets like GPCRs and other transmembrane proteins where recombinant expression or isolation may alter the native binding characteristics.
- Complete kinetic analysis on live cells: Unlike endpoint assays (e.g., flow cytometry or ELISA) that provide only a snapshot, scIC captures real-time binding and unbinding events. This enables accurate ka, kd, and KD measurements that reveal binding stability and residence times essential for therapeutic dosing.
- Exceptional sensitivity: scIC’s sensitivity enables detection of low-abundance targets (as low as 1,000 molecules per cell) and is compatible with a broad range of analytes, from small molecules to nanoparticles, peptides, and even viruses.
- Dual-colour and FRET capabilities: scIC’s dual-colour fluorescence and FRET detection capture real-time fluorescence signals, providing valuable data on fast binding events, particularly for small molecules and specific receptor interactions.
- Comprehensive cell target analysis: Traditional biosensors, like SPR and BLI, are limited by shallow detection depths and less effective cell immobilisation, which restricts cell-based measurements. scIC, with its 30 μm detection depth, captures binding events across the cell surface for a more complete analysis.
- Multiphasic binding analysis: scIC uses biphasic fit models to distinguish complex, multiphasic interactions, making it invaluable for studies on biologics and antibodies, where multivalent binding often occurs.

The heliXcyto biosensor.
SPR and scIC are essential for drug development kinetics
Together, SPR and scIC provide a comprehensive view of binding kinetics — from molecular-level interactions to cellular responses.
- Full data spectrum: While SPR delivers detailed kinetic parameters at the molecular level, scIC adds biological relevance by contextualising these interactions in a cellular setting.
- Predictive power for clinical outcomes: By combining SPR and scIC data, researchers gain a more complete picture of therapeutic binding properties, improving the predictability of clinical outcomes, therapeutic potency, and potential off-target effects.
- Accelerated, data-driven development: SPR quickly screens and ranks lead candidates, while scIC offers in-depth insights into the cellular effects of these candidates. Together, they support more informed decision-making early in the development process.
Join our community of curious minds on LinkedIn
🗓️ Stay informed with our monthly scientific newsletter, published on LinkedIn on the last Wednesday of each month.
These editions bring you the latest in drug development breakthroughs, industry trends, and expert insights from the brilliant minds at RoukenBio.
Subscribe today on LinkedInDiscover our binding kinetics capabilities
Our dual-technology platform combines SPR and scIC, providing a streamlined, robust approach for developing biologics, immunotherapies, or small molecules.
Learn more
