Biosimilars
With extensive experience in characterising antibody and antibody-like biosimilars, we offer expert support in designing analytical similarity assessments to navigate the regulatory landscape and ensure successful biosimilar development. This hinges on timely decisions and experienced partners.

Biosimilar Characterisation Services: Binding, Function, and Regulatory Confidence
Biosimilars are biological medicines that are highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, purity, or potency. Unlike generic drugs, which are chemically synthesised and identical to their reference products, biosimilars are derived from living systems and require a more complex demonstration of similarity.
Biosimilars play a critical role in expanding patient access to life-changing biologics and reducing healthcare costs while upholding the same standards of safety, efficacy, and quality as originator products. At RoukenBio, we’re proud to support this mission by empowering biosimilar developers with robust, comparative data that demonstrates biological activity and supports regulatory confidence.
Explore example binding and functional assay packages for key biosimilars
Each downloadable pack highlights the assays employed for binding and functional analytical similarity assessments for key biosimilars, including reporter assays, blocking and neutralisation formats, SPR binding, Fc‑receptor interactions and mechanism of action aligned functional readouts. These decks provide illustrative assay examples and representative data excerpts, giving you clear insight into the breadth of assays our team can support across biosimilar development.
These Assays Can Be Used To:
- Directly define and confirm critical quality attributes (CQAs) related to biological activity.
- Confirm the mechanism of action (e.g., receptor binding, neutralization).
- Help justify reduced reliance on animal and clinical studies.
- Investigate observed differences in structure or function.
- Enable extrapolation across multiple therapeutic indications.
At RoukenBio, we design and execute sensitive, MOA-reflective assays, including Fab and Fc binding, ADCC, CDC, and bioassays to meet regulatory expectations and de-risk development.
What our scientists say:
“I’m passionate about designing functional assays that do more than measure activity, they enable meaningful comparisons. In biosimilar development, it’s all about showing how two molecules behave side by side. That’s where comparative analysis comes in, and it’s where our assays make the difference.”
 | Amy Graham Study Manager |
Key Components of Biosimilar Development
Biosimilars are approved through a stepwise, totality-of-evidence approach defined by regulatory agencies such as the FDA and EMA including:
Analytical Characterisation
This is the foundation of biosimilar development. It involves detailed physicochemical and structural analyses to compare the biosimilar and reference product. Techniques include mass spectrometry, chromatography, and orthogonal methods to assess attributes like glycosylation, aggregation, and charge variants.
In Vitro Functional Assays
Regulatory agencies expect biosimilar developers to demonstrate that the proposed product matches the reference product not only in structure, but also in biological function. These assays are often the most sensitive tools available for detecting clinically relevant differences, and they directly inform regulatory decisions on biosimilarity.
Clinical Studies
These include pharmacokinetic (PK), pharmacodynamic (PD), immunogenicity, and, if needed, comparative efficacy trials. The extent of clinical data required depends on the strength of the analytical and functional evidence. A robust in vitro package can significantly reduce the clinical burden.
Our Capabilities: Binding and Functional Expertise
RoukenBio specialises in the comparative analytical and functional characterisation of biosimilars, with a focus on assays that reflect mechanism of action and regulatory expectations.
Fab Binding
FcγR, FcRn and C1q Binding
Fab-Mediated Functional Activity
Fc-Mediated Functional Activity
Advanced Functional Assays
Fab Binding
Fab binding assays are essential for demonstrating target engagement, a critical quality attribute in biosimilar development. These assays confirm that the biosimilar binds its intended antigen with comparable affinity and kinetics to the reference product. At RoukenBio, we use high-sensitivity platforms such as SPR, flow cytometry, single-cell interaction cytometry and ELISA to deliver robust, quantitative data that supports analytical similarity and mechanism-of-action alignment.
Fab Binding
Fab binding assays are essential for demonstrating target engagement, a critical quality attribute in biosimilar development. These assays confirm that the biosimilar binds its intended antigen with comparable affinity and kinetics to the reference product. At RoukenBio, we use high-sensitivity platforms such as SPR, flow cytometry, single-cell interaction cytometry and ELISA to deliver robust, quantitative data that supports analytical similarity and mechanism-of-action alignment.

FcγR, FcRn and C1q Binding
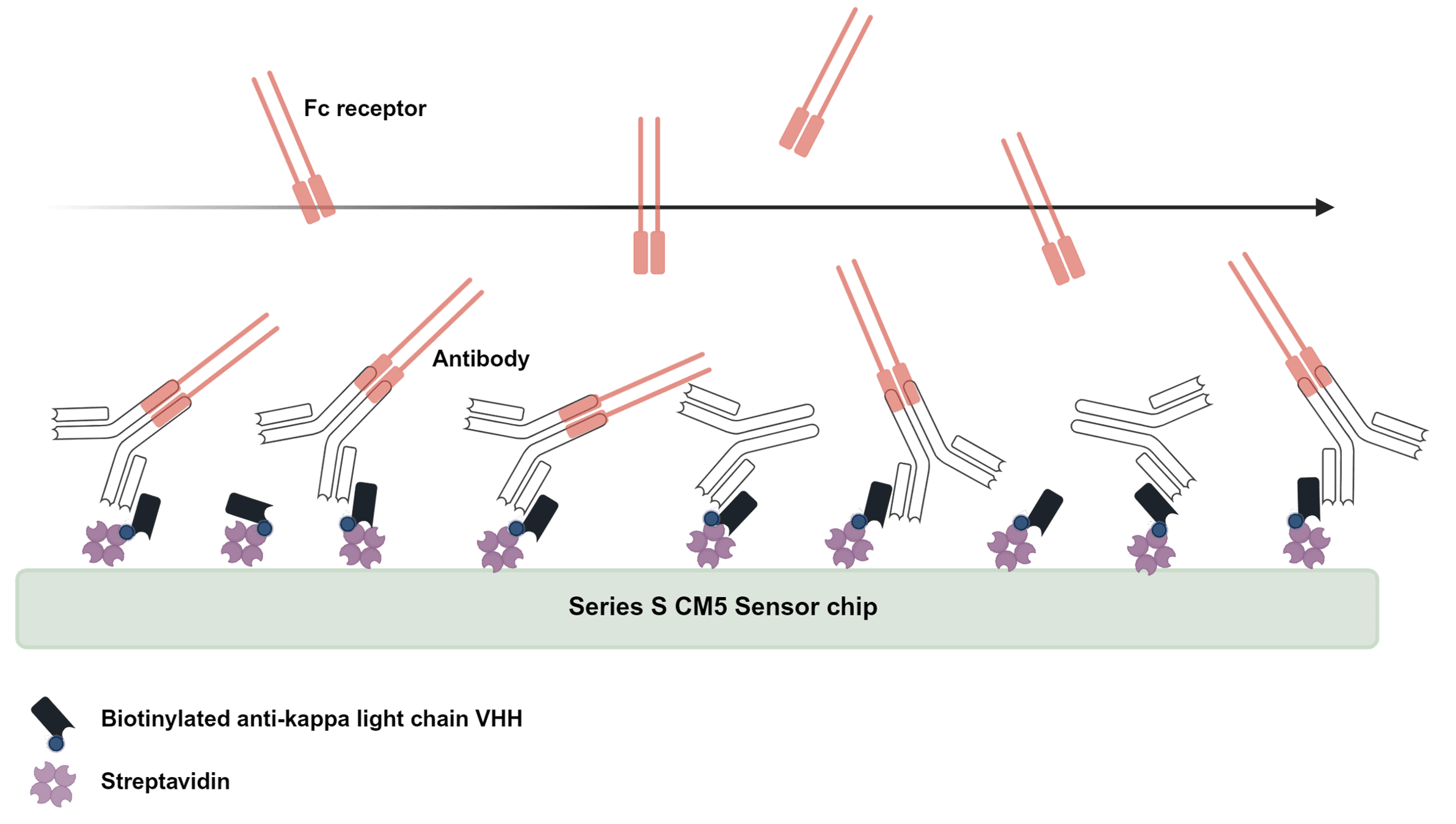
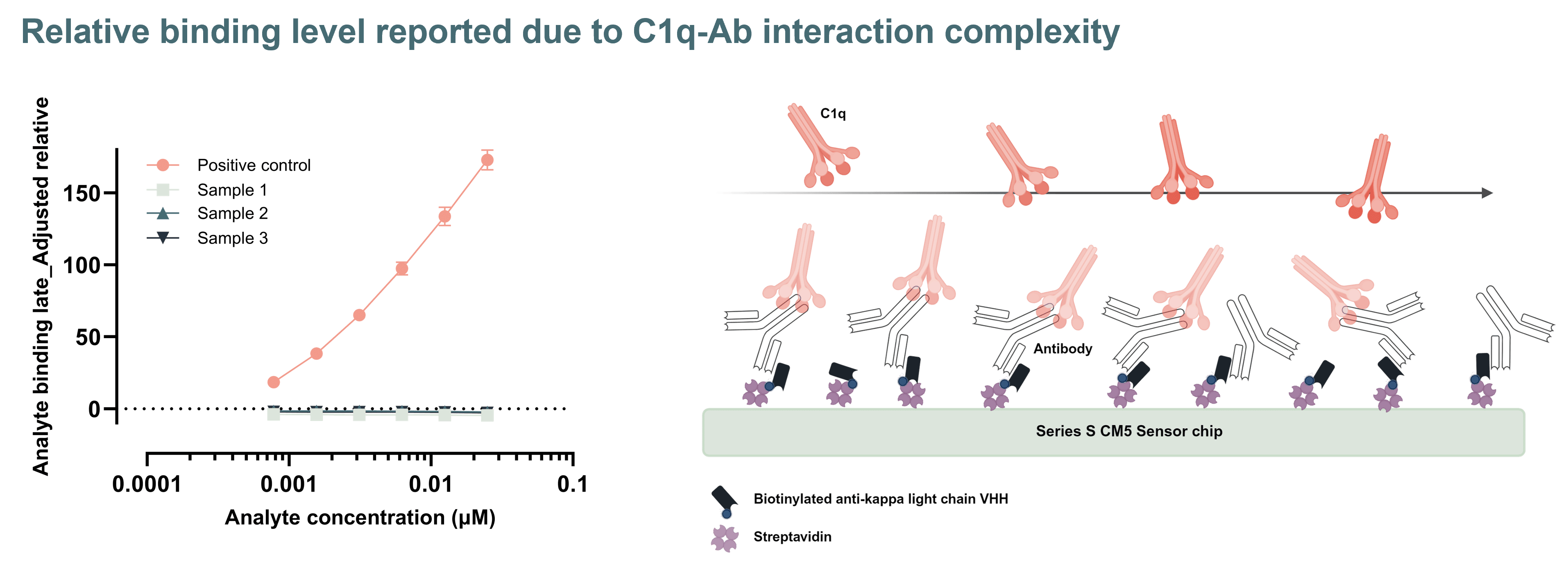
Fcγ receptor, FcRn and C1q binding assays are essential for assessing effector function comparability in biosimilar development. These assays evaluate interactions with FcγRI, FcγRIIa/b, FcγRIIIa/b, FcRn, and C1q, key mediators of immune effector functions such as ADCC, ADCP and CDC as well as half-life. At RoukenBio, we have particular expertise in demonstrating both the presence and the absence of binding activity, which is increasingly important for biosimilars engineered to lack effector function. Even when no binding is expected, regulators require sensitive, well-controlled assays to confirm this, making assay design and execution critical to a successful similarity assessment.
Fab-Mediated Functional Activity
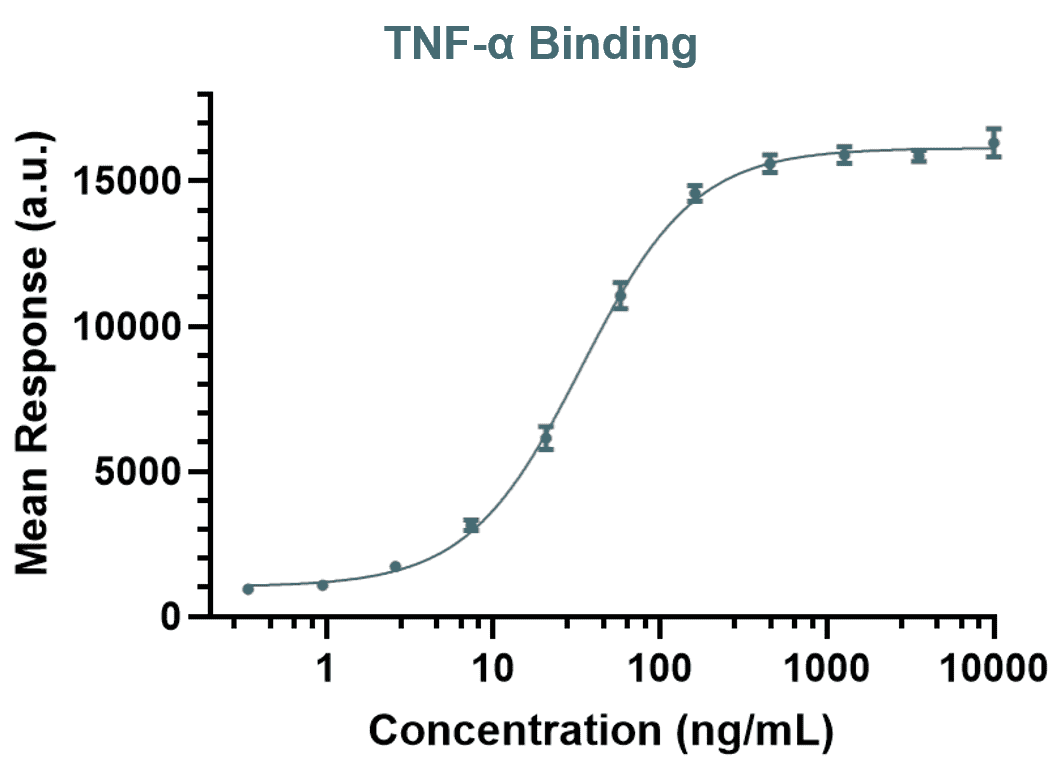
Fab-mediated functional activity assays are essential for demonstrating that a biosimilar performs its intended biological role. These assays evaluate functions such as target neutralisation, receptor blockade, and signal inhibition which are core aspects of the mechanism of action for many therapeutic antibodies. At RoukenBio, we design MOA-reflective assays that are tailored to the reference product’s biology and regulatory expectations. These assays play a critical role in defining potency, confirming analytical similarity, and supporting extrapolation across therapeutic indications.
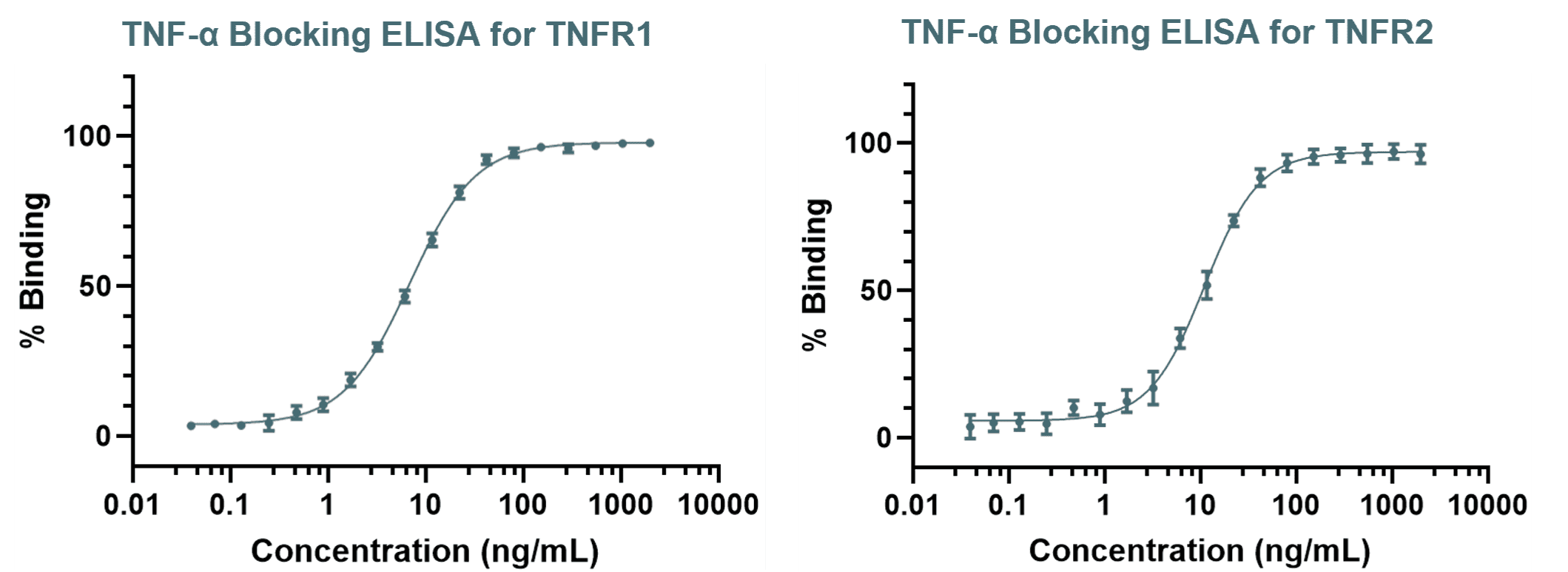
Fc-Mediated Functional Activity
Fc-mediated functional activity assays are essential for characterising the immune effector functions of biosimilars, including ADCC, CDC, and ADCP. These functions are often central to the therapeutic effect of monoclonal antibodies, and their accurate assessment is critical to demonstrating biosimilarity. At RoukenBio, we also specialise in confirming the absence of effector function, an increasingly common requirement for biosimilars engineered to be ‘silent’. Even when no activity is expected, regulators require sensitive, well-controlled assays to verify this, making assay design and execution vital to a successful analytical similarity assessment.
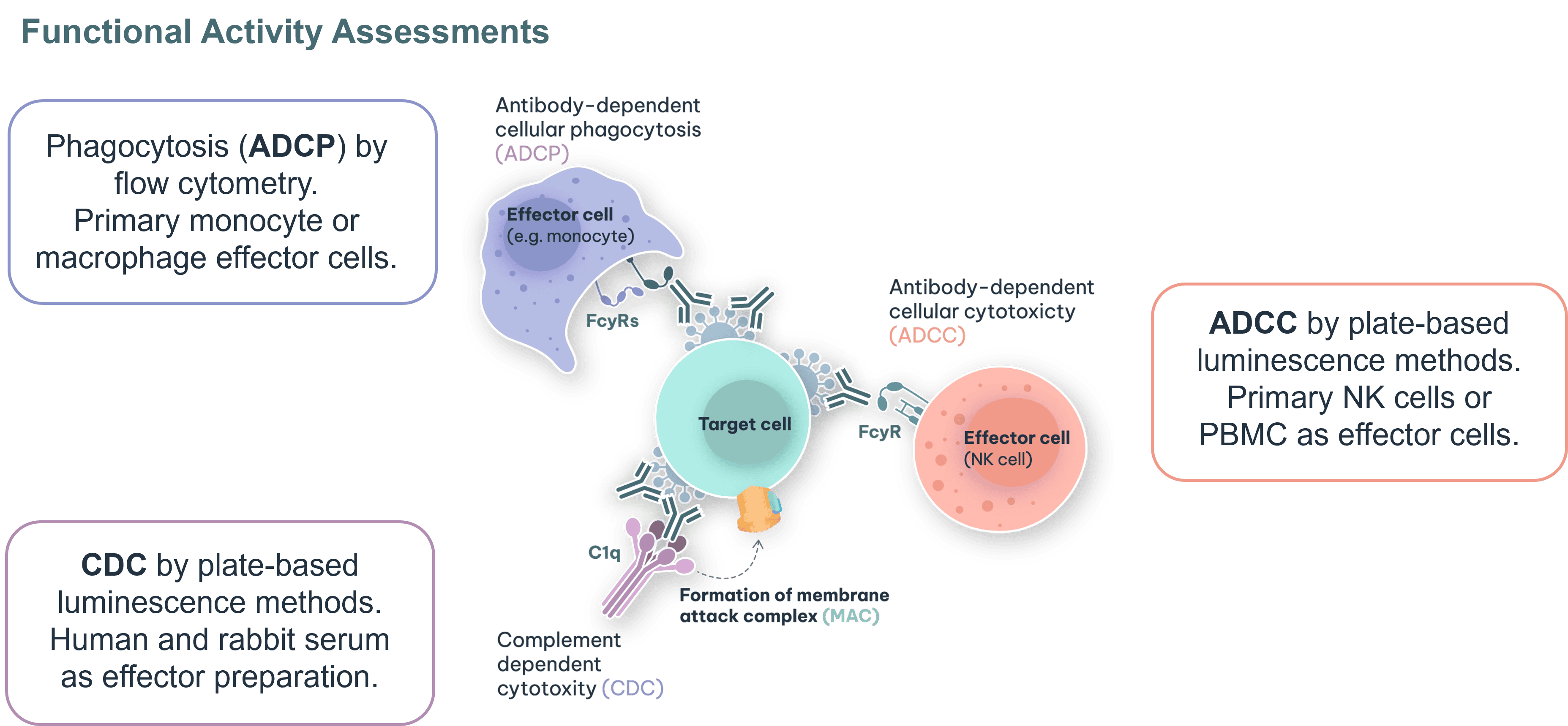
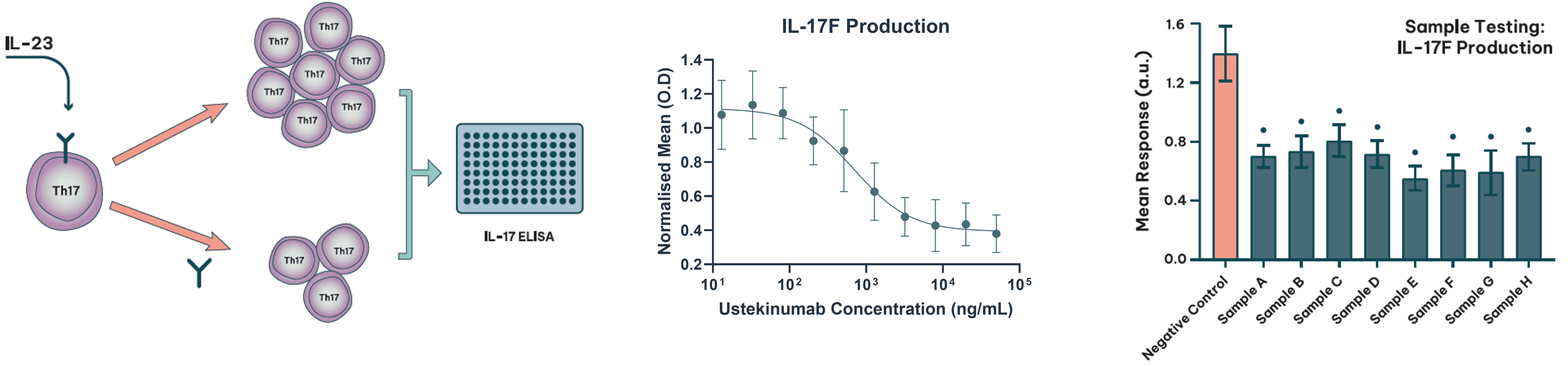
Advanced Functional Assays
Advanced functional assays provide deeper insight into biosimilar performance in biologically relevant systems. These include PD-linked assays, co-culture models, and primary cell-based systems that reflect the therapeutic context more closely than standard formats. Regulatory expectations recognise that not all assays must be quantitative. In some cases, particularly with complex or biologically variable systems, an assay may not be precise enough for formal statistical comparison, yet still provides meaningful insight when evaluated qualitatively or through graphical comparison. What matters is that the assay is scientifically justified, appropriately sensitive, and fit for its intended purpose. This is especially relevant when multiple orthogonal methods are used to assess the same attribute, allowing complementary data to build a robust picture of biosimilar performance.
At RoukenBio, we offer access to a wide range of primary human cell types, including large-lot cryopreserved PBMCs and isolated cells from both healthy and disease-state donors. This enables us to design assays that reflect real-world biology and support biosimilar developers in strengthening the totality-of-evidence package, especially when standard methods may not fully capture the biosimilar’s functional profile.
Our Quality Management System
At RoukenBio, we operate a robust Quality Management System (QMS) designed to support the scientific integrity, traceability, and reproducibility of our work. Our quality framework is designed to meet the needs of biosimilar developers and regulators, aligning with GxP principles, without requiring formal accreditation.
Our QMS includes documented procedures for:
- Documentation, change control, and good data practices
- Sample, material, and biological reagent management
- Equipment qualification, monitoring, and environmental control
- Deviation, CAPA, and contamination management
- Risk assessment, internal audits, and data integrity
- Training, supplier oversight, and customer study management
- Data storage, retention, and traceability
These systems ensure that our binding and functional assays are developed, executed, and reported with the rigour required to support regulatory submissions. Whether you're generating exploratory data or building a formal analytical similarity package, you can trust that our processes are designed to deliver high-quality, defensible results.
What our scientists say:
“I enjoy developing and qualifying assays that biosimilar developers can rely on. Whether it’s for early-stage exploration or formal similarity assessments, my goal is to ensure our methods are robust, reproducible, and fit for purpose so the data they generate stands up to scrutiny.”
 | Colin Forman Study Manager |
Our Experience in Biosimilar Development
At RoukenBio, we’ve supported biosimilar developers across a wide range of therapeutic areas and molecular targets. Our experience spans 18 biosimilar originator products, each with its own regulatory, scientific, and technical challenges.
Our team has been involved in biosimilar characterisation since 2012.
We’ve delivered hundreds of binding and functional studies, supporting both early-stage development and formal analytical similarity packages.
We’ve worked on biosimilars to some of the most clinically significant biologics, including:
1. Checkpoint Inhibitor Therapies
Nivolumab, Pembrolizumab – Block PD-1 to enhance T cell responses in cancer.
2. VEGF-Targeted Therapies
Bevacizumab, Aflibercept – Inhibit VEGF to block tumor angiogenesis.
3. TNF-Targeted Therapies
Adalimumab, Infliximab, Etanercept, Golimumab – Neutralize TNF-α to reduce inflammation in autoimmune diseases.
4. Interleukin-Targeted Therapies
Tocilizumab, Ustekinumab, Risankizumab, Guselkumab, Dupilumab, Secukinumab – Block ILs or their receptors to modulate immune responses.
5. CD20-Targeted Therapies
Rituximab, Ocrelizumab – Deplete B cells by targeting CD20 in cancer and autoimmune disease.
6. HER2-Targeted Therapies
Trastuzumab, Pertuzumab – Inhibit HER2 signaling in HER2-positive cancers.
7. RANKL-Targeted Therapy
Denosumab – Blocks osteoclast activity to reduce bone resorption.
8. RSV-Targeted Therapy
Palivizumab – Prevents RSV infection by targeting the viral F protein.
9. IgE – Targeted Therapy
Omalizumab – Inhibits binding of IgE to reduce magnitude of allergic reactions.
10. TSLP‑Targeted Therapy
Tezepelumab – Blocks TSLP to reduce airway inflammation in severe asthma.
What our clients say:
“We were impressed right away by the team’s depth of knowledge and appreciated their input on our proposed experimental plans; the RoukenBio team excelled at communication and executing the work. I would highly recommend working with RoukenBio.”
 | Dr Claudia Wiza Manager, Functional Assays |
Explore our Biosimilar capabilities
Get an in-depth look at our biosimilar expertise through our detailed slide deck, featuring sample data and a real-world biosimilar case study.
Access our technical slides






